

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM31896 Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 21.2HCl
SMILES: Clc1ccc(cc1)-c1nc2ccc(cc2c2OCCCc12)C(=O)NCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12
InChI Key: InChIKey=DYQPWUWERYOCLA-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31896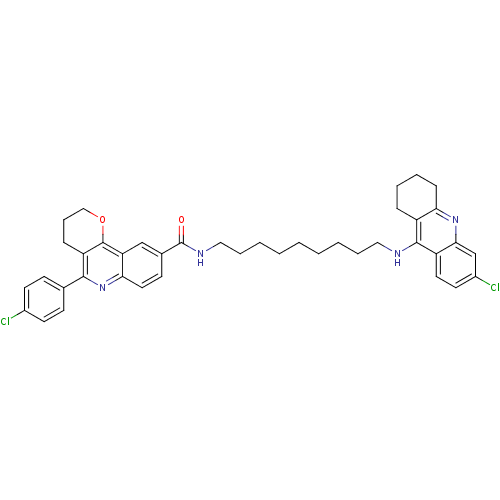 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 21....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.9 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM31896 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 21....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description BChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using BChE from human serum and butyrylthiocholine as substrat... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31896 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 21....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||