

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Oc1ccc(CCCCCC(=O)N2CCOC2=O)cc1
InChI Key: InChIKey=DHWUVTVUAVGPBO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM319818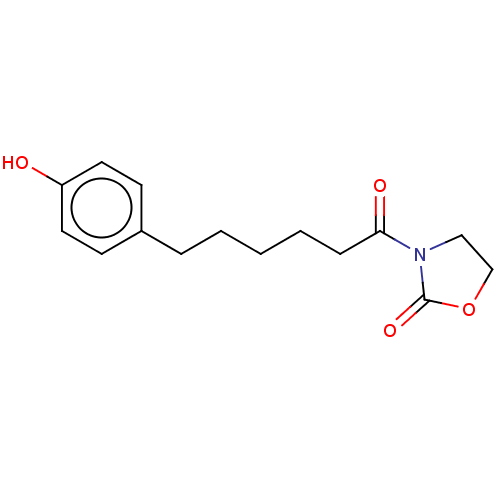 (US10174015, Compound 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Xiamen University US Patent | Assay Description In the Experimental example, endocannabinoid hydrolases used were Fatty Acid Amide Hydrolase (FAAH) and N-acylethanolamide hydrolyzing acid amidase (... | US Patent US10174015 (2019) BindingDB Entry DOI: 10.7270/Q2M047JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM319818 (US10174015, Compound 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat NAAA expressed in HEK293 cells using heptadecenoylethanolamide as substrate after 30 mins by HPLC-MS/MS analysis | Eur J Med Chem 139: 214-221 (2017) Article DOI: 10.1016/j.ejmech.2017.08.004 BindingDB Entry DOI: 10.7270/Q2FR007C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Rattus norvegicus (Rat)) | BDBM319818 (US10174015, Compound 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat acid ceramidase expressed in HEK293 cells using N-lauroylceramide as substrate after 30 mins by LC-MS analysis | Eur J Med Chem 139: 214-221 (2017) Article DOI: 10.1016/j.ejmech.2017.08.004 BindingDB Entry DOI: 10.7270/Q2FR007C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM319818 (US10174015, Compound 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Xiamen University US Patent | Assay Description In the Experimental example, endocannabinoid hydrolases used were Fatty Acid Amide Hydrolase (FAAH) and N-acylethanolamide hydrolyzing acid amidase (... | US Patent US10174015 (2019) BindingDB Entry DOI: 10.7270/Q2M047JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||