

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM33413 benzo[7]annulene-8-carboxylic acid, 5
SMILES: OC(=O)c1cc(O)c(=O)c2c(O)c(O)ccc2c1
InChI Key: InChIKey=XCSLJQFPTAZKDV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Reverse Transcriptase RNase H (Human immunodeficiency virus 1) | BDBM33413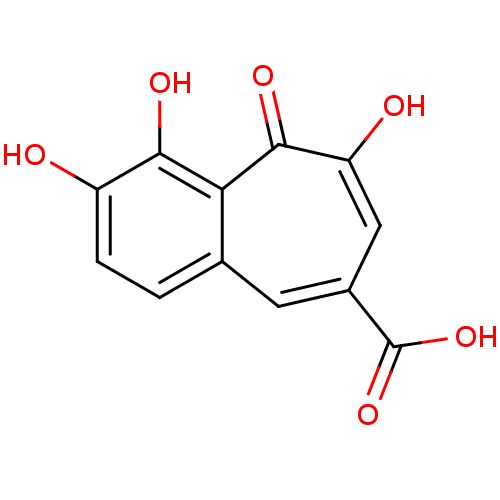 (benzo[7]annulene-8-carboxylic acid, 5) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cysteine protease ATG4B isoform a (Homo sapiens (Human)) | BDBM33413 (benzo[7]annulene-8-carboxylic acid, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of wild type recombinant Atg4B (unknown origin) expressed in Escherichia coli BL21 DE3 using N-terminal His6-tagged LC3B-PLA2 as substrate... | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens) | BDBM33413 (benzo[7]annulene-8-carboxylic acid, 5) | PDB KEGG GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam University Curated by ChEMBL | Assay Description Inhibition of recombinant human SHP2 expressed in Escherichia coli using 2P-IRS1 peptide as substrate after 1 hr by microplate reader analysis | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM33413 (benzo[7]annulene-8-carboxylic acid, 5) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at GPR35 receptor in human U2OS cells coexpressing Gal4-VP16-TEV assessed as beta arrestin translocation after 5 hrs by beta lactmas... | ACS Med Chem Lett 3: 165-169 (2012) Article DOI: 10.1021/ml2003058 BindingDB Entry DOI: 10.7270/Q2DB82W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM33413 (benzo[7]annulene-8-carboxylic acid, 5) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Desensitization of GPR35 receptor in human HT-29 cells assessed as inhibition of zaprinast-induced dynamic mass redistribution after 10 mins | ACS Med Chem Lett 3: 165-169 (2012) Article DOI: 10.1021/ml2003058 BindingDB Entry DOI: 10.7270/Q2DB82W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM33413 (benzo[7]annulene-8-carboxylic acid, 5) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at GPR35 receptor in human HT-29 cells after 10 mins by dynamic mass redistribution assay | ACS Med Chem Lett 3: 165-169 (2012) Article DOI: 10.1021/ml2003058 BindingDB Entry DOI: 10.7270/Q2DB82W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||