

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM40135 (3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylidene)-1-benzopyran-2,7-dione::(3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylidene)chromene-2,7-dione::(3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylidene)chromene-2,7-quinone::6-Hexyl-7-hydroxy-3-(5-phenyl-[1,3,4]oxadiazol-2-yl)-chromen-2-one::MLS000068623::SMR000122957::cid_6737494
SMILES: CCCCCCC1=Cc2c\c(=C3\NN=C(O3)c3ccccc3)c(=O)oc2=CC1=O
InChI Key: InChIKey=JTSFXCSVRWDWED-RELWKKBWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM40135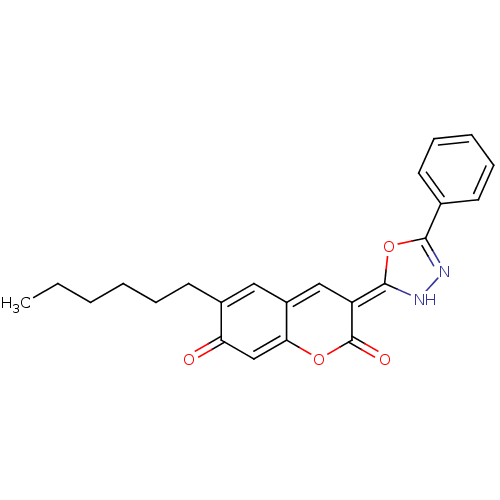 ((3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylid...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: John A. Katzenellenbogen, ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2QJ7FQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated calcium channel subunit alpha Cav2.2 (Homo sapiens (Human)) | BDBM40135 ((3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 9.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute Network... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2PZ578V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| streptokinase A precursor (Streptococcus pyogenes M1 GAS) | BDBM40135 ((3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Group A streptococcus, GAS, streptokinase, expression, virulence, inhibition, dose response, EC50 Assay Overview: The goal of this assa... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2736PBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM40135 ((3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q25M647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic peptidase activating factor 1 (Homo sapiens (Human)) | BDBM40135 ((3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2JS9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic peptidase activating factor 1 (Homo sapiens (Human)) | BDBM40135 ((3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2F18X8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1H (Homo sapiens (Human)) | BDBM40135 ((3E)-6-hexyl-3-(5-phenyl-3H-1,3,4-oxadiazol-2-ylid...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters Curated by PubChem BioAssay | Assay Description Assay Provider: Xinmin Xie Assay Provider Affiliation: Bioscience Division, SRI International, Menlo Park, CA Grant Title: HTS Assay for Cav3 T-Type ... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2B856KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||