

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1nnn(C)c1-c1cc2n([C@@H](C3CCOCC3)c3ccccc3)c3cc(cnc3c2cc1Cl)C(C)(C)O
InChI Key: InChIKey=JVPYSHCVNWGQBI-GDLZYMKVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myc proto-oncogene protein (Homo sapiens (Human)) | BDBM445584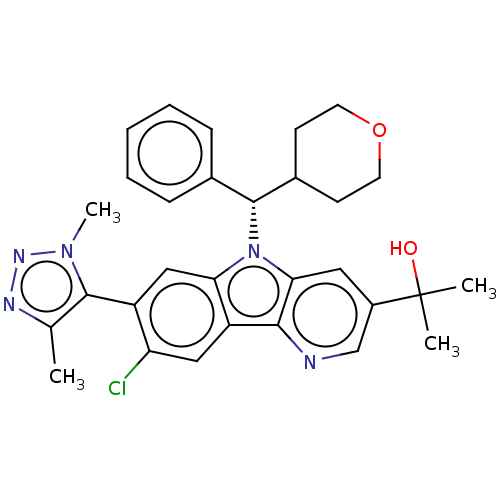 (US10683290, Example 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of c-MYC (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128376 BindingDB Entry DOI: 10.7270/Q2KS6WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477] (Homo sapiens (Human)) | BDBM445584 (US10683290, Example 77) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description All assay components were dissolved in buffer composition 20 mM Hepes pH 7.5, 150 mM NaCl, 5 mM DTT, 0.005% Tween 20, and 100 ug/ml BSA for BRD4 (1-4... | US Patent US10683290 (2020) BindingDB Entry DOI: 10.7270/Q25D8VWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||