

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1c(Nc2nc(nc3cc(CN4CC[C@@H](O)C4)cnc23)C2CC2)cccc1-c1cccc(-c2nc3cc(CN4CC[C@H](C4)C(O)=O)cc(C#N)c3o2)c1C
InChI Key: InChIKey=ARRUIUNMLVKZBV-BVRKHOPBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446475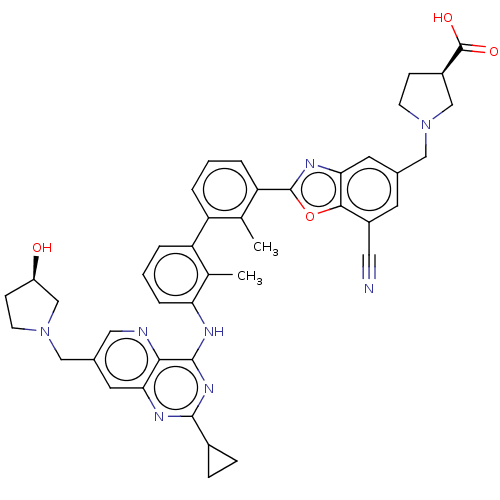 ((R)-1-((7-cyano-2-(3′-(2-cyclopropyl-7-(((R)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446475 ((R)-1-((7-cyano-2-(3′-(2-cyclopropyl-7-(((R)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM446475 ((R)-1-((7-cyano-2-(3′-(2-cyclopropyl-7-(((R)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description PD-L1 aAPC/CHO-Klcells (Promega) were maintained in F-12 medium with addition of 10% FBS, 200 μg/ml Hygromycin B, 250 μg/ml Geneticin (G418... | US Patent US10669271 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446475 ((R)-1-((7-cyano-2-(3′-(2-cyclopropyl-7-(((R)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||