 Found 10 hits for monomerid = 48319
Found 10 hits for monomerid = 48319 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM48319
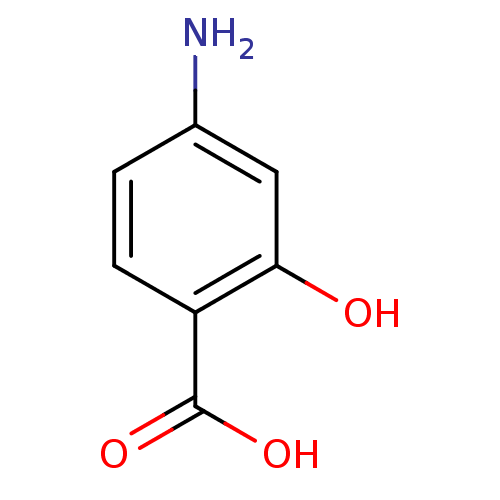
(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 esterase activity by noncompetitive Lineweaver-Burk plot |
Bioorg Med Chem 16: 9101-5 (2008)
Article DOI: 10.1016/j.bmc.2008.09.028
BindingDB Entry DOI: 10.7270/Q2GB23W2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 esterase activity by non-competitive Lineweaver-Burk plot |
Bioorg Med Chem 16: 9101-5 (2008)
Article DOI: 10.1016/j.bmc.2008.09.028
BindingDB Entry DOI: 10.7270/Q2GB23W2 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+6 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Protein-tyrosine phosphatase 1B (PTP 1B) site 2 Ligands were identified using [13C]-labeled protein and their Dissociation Constants were determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant of the compound against protein tyrosine phosphatase PTB1B receptor was determined |
J Med Chem 46: 4232-5 (2003)
Article DOI: 10.1021/jm034122o
BindingDB Entry DOI: 10.7270/Q2BP0264 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | n/a | 1.20E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 esterase activity by spectrophotometry |
Bioorg Med Chem 16: 9101-5 (2008)
Article DOI: 10.1016/j.bmc.2008.09.028
BindingDB Entry DOI: 10.7270/Q2GB23W2 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+6 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Estradiol receptor beta (ERβ)
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PCBioAssay
| n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
| Assay Description
NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: John A. Katzenellenbogen, ... |
PubChem Bioassay (2007)
BindingDB Entry DOI: 10.7270/Q2WD3Z0J |
More data for this
Ligand-Target Pair | |
Estradiol receptor beta (ERβ)
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PCBioAssay
| n/a | n/a | 6.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
| Assay Description
NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: John A. Katzenellenbogen, ... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2CF9NHH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM48319

(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 esterase activity by spectrophotometry |
Bioorg Med Chem 16: 9101-5 (2008)
Article DOI: 10.1016/j.bmc.2008.09.028
BindingDB Entry DOI: 10.7270/Q2GB23W2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data