

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM4929 (2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetamido-3,4-dihydro-2H-pyran-6-carboxylic acid::CHEMBL295490::carboxamide deriv. 4g
SMILES: [H][C@]1(OC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O)C(=O)N(CCC)CCC
InChI Key: InChIKey=ZXZWQHMARLSKTR-CYZMBNFOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase A (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4929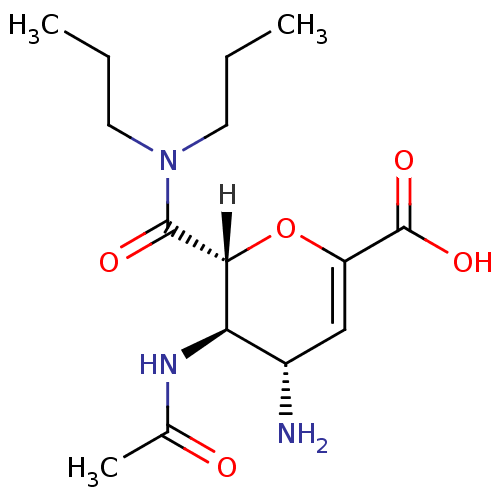 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase B (Influenza B virus) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase A (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza sialidase was determined by measuring the ability to inhibit the hydrolysis of MUN by A/Aichi virus grown in hen eggs | Bioorg Med Chem Lett 6: 1805-1808 (1996) Article DOI: 10.1016/0960-894X(96)00318-6 BindingDB Entry DOI: 10.7270/Q2T1544R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase B (Influenza B virus (B/Lee/40)) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza sialidase was determined by measuring the ability to inhibit the hydrolysis of MUN by B Victoria virus grown in hen eggs | Bioorg Med Chem Lett 6: 1805-1808 (1996) Article DOI: 10.1016/0960-894X(96)00318-6 BindingDB Entry DOI: 10.7270/Q2T1544R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Inhibition of influenza virus neuraminidase | Eur J Med Chem 43: 569-76 (2008) Article DOI: 10.1016/j.ejmech.2007.04.011 BindingDB Entry DOI: 10.7270/Q2C53PNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase B (Influenza B virus (B/Lee/40)) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza B sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase B (Influenza B virus (B/Lee/40)) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza B sialidase (Victoria) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase A (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against influenza A sialidase (Aichi) | Bioorg Med Chem Lett 11: 669-73 (2001) BindingDB Entry DOI: 10.7270/Q26M37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase A (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4929 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||