

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50002751 11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl]-6,11-dihydro-dibenzo[b,e]oxepine-2-carboxylic acid::CHEMBL324364
SMILES: OC(=O)c1ccc2OCc3ccccc3C(SCCNS(=O)(=O)\C=C\c3ccccc3)c2c1
InChI Key: InChIKey=QTZKOUSSPHZWGR-NTCAYCPXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002751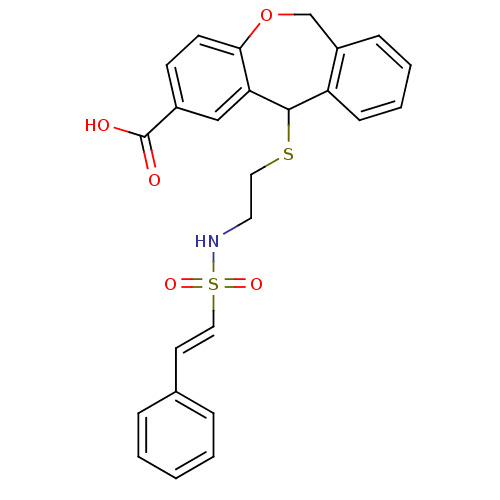 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of the thromboxane A2 receptor assayed by binding to guinea pig platelets using [3H]-U-46,619 as radioligand | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for its binding affinity at Thromboxane A2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid TP receptor (Homo sapiens (Human)) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for its binding affinity at Thromboxane A2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets ... | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for its binding affinity at Thromboxane A2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of Shh signaling in mouse Shh-light2 cells by Gli-dependent firefly luciferase reporter gene assay | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against thromboxane A2 synthase binding to bovine platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against thromboxane A2 synthase binding to bovine platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Rattus norvegicus) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against Thromboxane A2 synthase obtained from bovine platelet microsomes | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||