 Found 14 hits for monomerid = 50004774
Found 14 hits for monomerid = 50004774 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA)
(Homo sapiens (Human)) | BDBM50004774
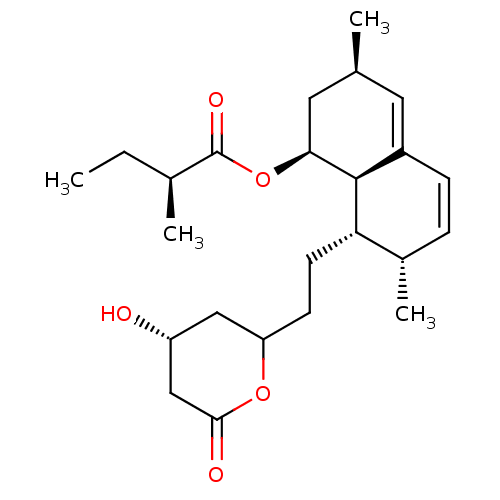
((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human HMGCoA reductase |
J Nat Prod 52: 153-161 (1989)
Article DOI: 10.1021/np50061a020
BindingDB Entry DOI: 10.7270/Q28052MW |
More data for this
Ligand-Target Pair | |
HMG-CoA reductase
(Rattus norvegicus (rat)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of HMG-CoA reductase of rat liver |
J Med Chem 33: 61-70 (1990)
BindingDB Entry DOI: 10.7270/Q22V2F4S |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA)
(Homo sapiens (Human)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
Inhibition of cellular HMG-CoA reductase in cultures of hepatic cells (HEP G2, a human hepatoma cell line) |
J Med Chem 33: 61-70 (1990)
BindingDB Entry DOI: 10.7270/Q22V2F4S |
More data for this
Ligand-Target Pair | |
HMG-CoA reductase
(Rattus norvegicus (rat)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG
Curated by ChEMBL
| Assay Description
Tested for inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 37: 3240-6 (1994)
BindingDB Entry DOI: 10.7270/Q25H7F9D |
More data for this
Ligand-Target Pair | |
HMG-CoA reductase
(Rattus norvegicus (rat)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibition of ACAT in Albino rabbit intestinal mucosa |
Bioorg Med Chem Lett 17: 1946-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.027
BindingDB Entry DOI: 10.7270/Q23J3CMT |
More data for this
Ligand-Target Pair | |
Integrin beta-2/Intercellular adhesion molecule-1 /Leukocyte adhesion glycoprotein LFA-1 alpha
(Homo sapiens (Human)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HUT78 cell adhesion to immobilized ICAM-1 protein |
Bioorg Med Chem Lett 14: 2483-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.006
BindingDB Entry DOI: 10.7270/Q2N58KT2 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA)
(Homo sapiens (Human)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in HepG2 cells |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
HMG-CoA reductase
(Mus musculus) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibitory activity against isolated enzyme HMG-CoA reductase |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
HMG-CoA reductase
(Rattus norvegicus (rat)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
HMG-CoA reductase
(Rattus norvegicus (rat)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for the HMG-CoA reductase inhibitory activity in a microsomal preparation |
Bioorg Med Chem Lett 7: 549-554 (1997)
Article DOI: 10.1016/S0960-894X(97)00065-6
BindingDB Entry DOI: 10.7270/Q2RB74NQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA)
(Homo sapiens (Human)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human HMGCoA reductase |
J Nat Prod 52: 153-161 (1989)
Article DOI: 10.1021/np50061a020
BindingDB Entry DOI: 10.7270/Q28052MW |
More data for this
Ligand-Target Pair | |
HMG-CoA reductase
(Rattus norvegicus (rat)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of HMG-CoA reductase (COR) in rats. |
J Med Chem 34: 367-73 (1991)
BindingDB Entry DOI: 10.7270/Q2J38RHG |
More data for this
Ligand-Target Pair | |
HMG-CoA reductase
(Rattus norvegicus (rat)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase from rat hepatocyte |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data