

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50006687 (S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6-yl)methyl)(prop-2-ynyl)amino)benzamido)pentanedioic acid::(S)-2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-ylmethyl)-prop-2-ynyl-amino]-benzoylamino}-pentanedioic acid::2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-ylmethyl)-prop-2-ynyl-amino]-benzoylamino}-pentanedioic acid::2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-ylmethyl)-prop-2-ynyl-amino]-benzoylamino}-pentanedioic acid ;hydrate(2H2O)::2-{4-[1-(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-ylmethyl)-prop-2-ynyl]-benzoylamino}-pentanedioic acid::CHEMBL434209
SMILES: Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1
InChI Key: InChIKey=PMTUUSDTAKQWQJ-NRFANRHFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50006687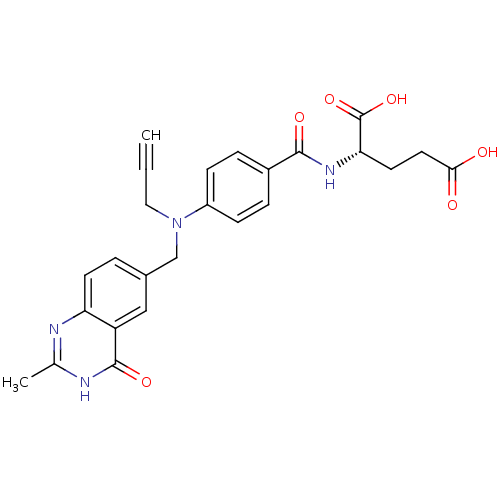 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Meerut Institute of Engineering and Technology Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase | Eur J Med Chem 45: 1560-71 (2010) Article DOI: 10.1016/j.ejmech.2009.12.065 BindingDB Entry DOI: 10.7270/Q2D50P61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells | J Med Chem 42: 3809-20 (1999) BindingDB Entry DOI: 10.7270/Q25M64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of partially purified L1210 thymidylate synthase (TS). | J Med Chem 34: 1594-605 (1991) BindingDB Entry DOI: 10.7270/Q22N52VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the compound against L1210 thymidylate synthase (TS) | J Med Chem 34: 2209-18 (1991) BindingDB Entry DOI: 10.7270/Q2D799D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase from L1210 cells | J Med Chem 35: 2321-7 (1992) BindingDB Entry DOI: 10.7270/Q2XW4HRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||