

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50010448 2,2'-Diimino-5,5'-diisopropyl-7,7'-dimethyl-2H,2'H-[8,8']bi[naphtho[1,8-bc]furanyl]-3,4,3',4'-tetraol::CHEMBL424250
SMILES: CC(C)c1c(O)c(O)c2C(=N)Oc3c(c(C)cc1c23)-c1c2OC(=N)c3c(O)c(O)c(C(C)C)c(cc1C)c23
InChI Key: InChIKey=RBFMXSCFUBMKHQ-UHFFFAOYSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactate dehydrogenase A (LDHA) (Homo sapiens (Human)) | BDBM50010448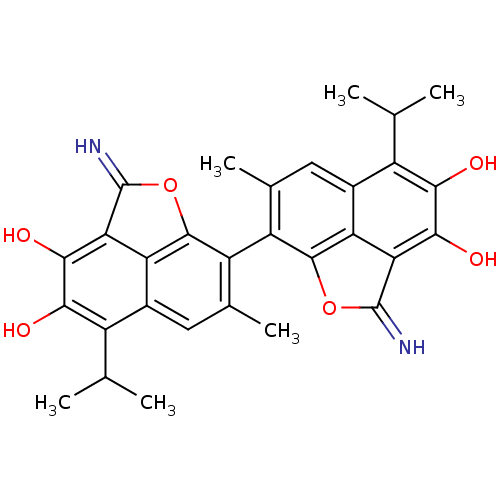 (2,2'-Diimino-5,5'-diisopropyl-7,7'-dimethyl-2H,2'H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO Curated by ChEMBL | Assay Description Competitive inhibition of human LDH5 in presence of NADH | J Med Chem 59: 487-96 (2016) BindingDB Entry DOI: 10.7270/Q2B27X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (AR) (Homo sapiens (Human)) | BDBM50010448 (2,2'-Diimino-5,5'-diisopropyl-7,7'-dimethyl-2H,2'H...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactate dehydrogenase B (LDHB) (Homo sapiens (Human)) | BDBM50010448 (2,2'-Diimino-5,5'-diisopropyl-7,7'-dimethyl-2H,2'H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO Curated by ChEMBL | Assay Description Competitive inhibition of human LDH1 in presence of NADH | J Med Chem 59: 487-96 (2016) BindingDB Entry DOI: 10.7270/Q2B27X41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||