

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50017876 2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylmethyl)-ethyl-amino]-benzoylamino}-pentanedioic acid::CHEMBL22632
SMILES: CCN(Cc1ccc2nc(N)[nH]c(=O)c2c1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O
InChI Key: InChIKey=QJODZQWWCMYPFD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50017876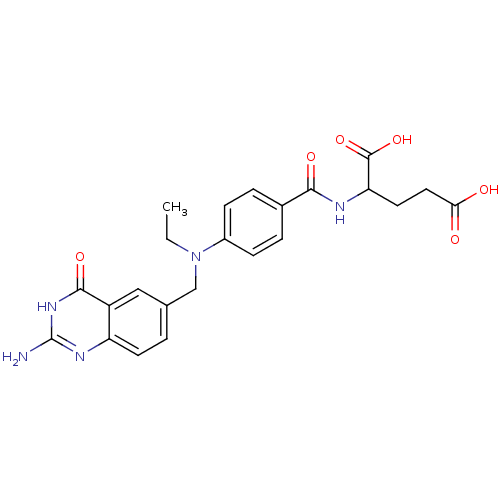 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase (DHFR) enzyme. | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GAR transformylase (Mus musculus) | BDBM50017876 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of GAR transformylase isolated from the murine lymphoma cell line L5178Y | J Med Chem 30: 1254-6 (1987) BindingDB Entry DOI: 10.7270/Q2VX0FHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate Synthase (TS) (Lactobacillus casei) | BDBM50017876 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||