

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50055660 1-amino-3-(4-(4-(2-(amino(iminio)methylamino)acetamido)-1-methyl-1H-pyrrole-2-carboxamido)-1-methyl-1H-pyrrole-2-carboxamido)propan-1-iminium::2N-(3-amino-3-iminopropyl)-4-[4-amino(imino)methylaminomethylcarboxamido-1-methyl-1H-2-pyrrolylcarboxamido]-1-methyl-1H-2-pyrrolecarboxamide::2N-(3-amino-3-iminopropyl)-4-[4-amino(imino)methylaminomethylcarboxamido-1-methyl-1H-2-pyrrolylcarboxamido]-1-methyl-1H-2-pyrrolecarboxamide(netropsin)::4-(2-Guanidino-acetylamino)-1-methyl-1H-pyrrole-2-carboxylic acid [5-(2-carbamimidoyl-ethylcarbamoyl)-1-methyl-2,3-dihydro-1H-pyrrol-3-yl]-amide::CHEMBL307767::NETROPSIN
SMILES: [#6]-n1cc(-[#7]-[#6](=O)-c2cc(-[#7]-[#6](=O)-[#6]\[#7]=[#6](\[#7])-[#7])cn2-[#6])cc1-[#6](=O)-[#7]-[#6]-[#6]-[#6](-[#7])=[#7]
InChI Key: InChIKey=IDBIFFKSXLYUOT-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UvrD helicase (PfUDN) (Plasmodium falciparum) | BDBM50055660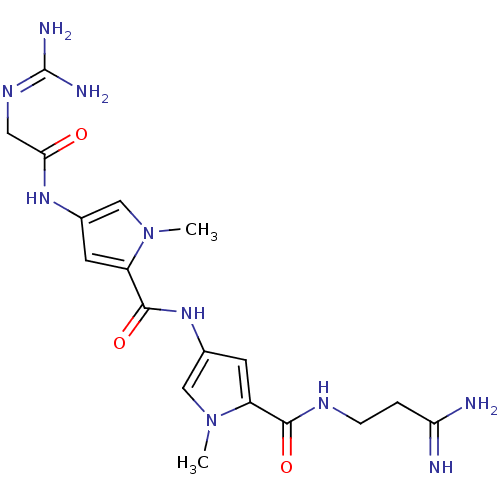 (1-amino-3-(4-(4-(2-(amino(iminio)methylamino)aceta...) | GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
International Centre for Genetic Engineering and Biotechnology | Assay Description The hydrolysis of ATP catalyzed by PfUDN was assayed by measuring the formation of Pi from [γ-32P] ATP. The reaction mixture of 10 μl conta... | BMC Biochem 15: 9 (2014) Article DOI: 10.1186/1471-2091-15-9 BindingDB Entry DOI: 10.7270/Q25H7F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UvrD helicase (PfUDN) (Plasmodium falciparum) | BDBM50055660 (1-amino-3-(4-(4-(2-(amino(iminio)methylamino)aceta...) | GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
International Centre for Genetic Engineering and Biotechnology | Assay Description Helicase assay was demonstrated using the purified fraction of PfUDN. The specially designed partial duplex substrate consisted of a 32P-labelled 47-... | BMC Biochem 15: 9 (2014) Article DOI: 10.1186/1471-2091-15-9 BindingDB Entry DOI: 10.7270/Q25H7F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase I (Topo I) (Homo sapiens (Human)) | BDBM50055660 (1-amino-3-(4-(4-(2-(amino(iminio)methylamino)aceta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibit supercoil relaxation property of topoisomerase I. | J Med Chem 40: 216-25 (1997) Article DOI: 10.1021/jm9605804 BindingDB Entry DOI: 10.7270/Q2F47PS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50055660 (1-amino-3-(4-(4-(2-(amino(iminio)methylamino)aceta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University | Assay Description Quantities of 0.2 mL of examined preparation (as control, 0.15 M NaCl), buffer, and 0.1 mL of enzyme solution were mixed together. The mixture was in... | J Enzyme Inhib Med Chem 25: 629-34 (2010) Article DOI: 10.3109/14756360903389872 BindingDB Entry DOI: 10.7270/Q2SB44NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50055660 (1-amino-3-(4-(4-(2-(amino(iminio)methylamino)aceta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University | Assay Description Quantities of 0.2 mL of examined preparation (as control, 0.15 M NaCl), buffer, and 0.1 mL of enzyme solution were mixed together. The mixture was in... | J Enzyme Inhib Med Chem 25: 629-34 (2010) Article DOI: 10.3109/14756360903389872 BindingDB Entry DOI: 10.7270/Q2SB44NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||