 Found 17 hits for monomerid = 50067533
Found 17 hits for monomerid = 50067533 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50067533
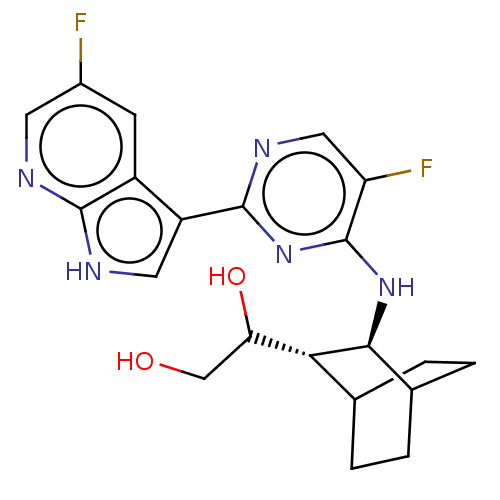
(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1/2
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SRC (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MET (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PLK (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50067533

(CHEMBL3401984)Show SMILES [H][C@]1(C(O)CO)C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wD:12.14,1.0,TLB:2:1:8.7:10.11,THB:13:12:8.7:10.11,(-.64,1.55,;.13,.87,;1.58,1.51,;2.6,.82,;1.69,3.04,;2.79,3.59,;1.39,,;2.86,.48,;2.23,-.94,;,-.67,;-.29,-2.25,;1.15,-1.65,;-1.3,.28,;-2.82,.5,;-3.77,-.71,;-3.2,-2.14,;-4.15,-3.35,;-5.68,-3.13,;-6.25,-1.7,;-5.3,-.49,;-5.76,.65,;-3.58,-4.78,;-4.4,-6.04,;-3.43,-7.23,;-2,-6.66,;-.62,-7.36,;.67,-6.49,;.57,-4.96,;1.59,-4.28,;-.83,-4.26,;-2.1,-5.13,)| Show InChI InChI=1S/C21H23F2N5O2/c22-12-5-13-14(7-25-19(13)24-6-12)20-26-8-15(23)21(28-20)27-18-11-3-1-10(2-4-11)17(18)16(30)9-29/h5-8,10-11,16-18,29-30H,1-4,9H2,(H,24,25)(H,26,27,28)/t10?,11?,16?,17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PKAa (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data