

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50068267 (E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-phenyl)-propenone::3-(4-hydroxy-2-methoxyphenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one::CHEMBL141530::Echinatin
SMILES: COc1cc(O)ccc1\C=C\C(=O)c1ccc(O)cc1
InChI Key: InChIKey=QJKMIJNRNRLQSS-WEVVVXLNSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50068267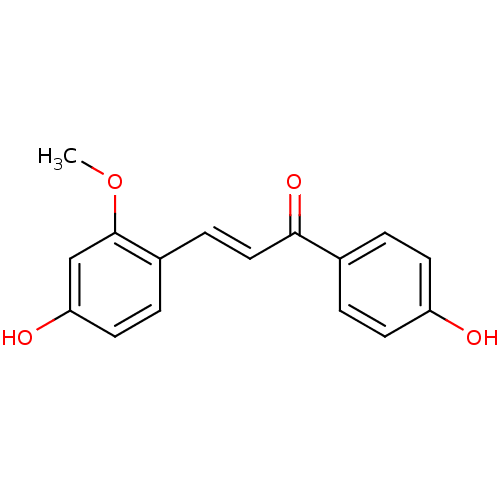 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B assessed as hydrolysis of p-nitrophenyl phosphate after 30 mins | Bioorg Med Chem Lett 19: 5155-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.054 BindingDB Entry DOI: 10.7270/Q24X58R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A H9N2 virus neuraminidase activity after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase activity after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant H1N1 swine influenza virus neuraminidase H274Y mutant activity expressed in HEK293T cells after 2 hrs by spectrof... | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of TDP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) using 5'-FAM-AGGATCTAAAAGACTT-BHQ-3' as substrate preincubated for 30 mi... | Bioorg Med Chem 28: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A H1N1 virus neuraminidase activity by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHA University Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using Z-FR-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3320-4 (2013) Article DOI: 10.1016/j.bmcl.2013.03.106 BindingDB Entry DOI: 10.7270/Q2PN98J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHA University Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using RR-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3320-4 (2013) Article DOI: 10.1016/j.bmcl.2013.03.106 BindingDB Entry DOI: 10.7270/Q2PN98J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50068267 ((E)-3-(4-Hydroxy-2-methoxy-phenyl)-1-(4-hydroxy-ph...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of wild type H1N1 swine influenza virus neuraminidase activity expressed in HEK293T cells after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||