

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50070010 4-((2R,3R)-1-Benzylcarbamoyl-3-methyl-4-oxo-azetidin-2-yloxy)-benzoic acid::CHEMBL122575
SMILES: C[C@@H]1[C@@H](Oc2ccc(cc2)C(O)=O)N(C(=O)NCc2ccccc2)C1=O
InChI Key: InChIKey=BRYGRWNTDBRNDJ-YVEFUNNKSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-chymotrypsin (Bos taurus (bovine)) | BDBM50070010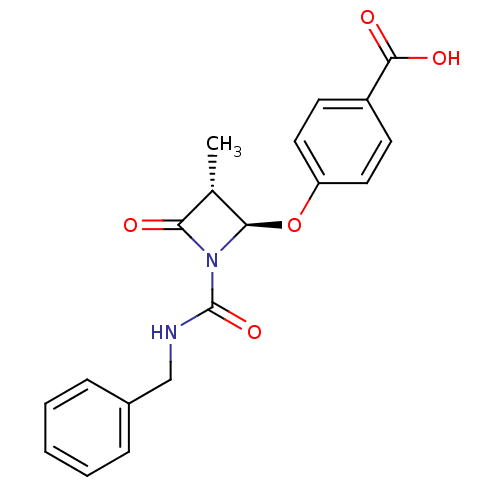 (4-((2R,3R)-1-Benzylcarbamoyl-3-methyl-4-oxo-azetid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit the mammalian Chymotrypsinogen by 50% was determined | Bioorg Med Chem Lett 8: 365-70 (1999) BindingDB Entry DOI: 10.7270/Q2HD7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human herpes virus 5 capsid protein P40 (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50070010 (4-((2R,3R)-1-Benzylcarbamoyl-3-methyl-4-oxo-azetid...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit the mutant alpha-Ala HCMV protease by 50% was determined | Bioorg Med Chem Lett 8: 365-70 (1999) BindingDB Entry DOI: 10.7270/Q2HD7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50070010 (4-((2R,3R)-1-Benzylcarbamoyl-3-methyl-4-oxo-azetid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit the mammalian Acetylcholinesterase by 50% was determined | Bioorg Med Chem Lett 8: 365-70 (1999) BindingDB Entry DOI: 10.7270/Q2HD7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||