

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50073752 (R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-thiophene-2-carbonyl}-amino)-2-methyl-pentanedioic acid::CHEMBL168069::LY-335580
SMILES: C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)s1)C(O)=O
InChI Key: InChIKey=KHOJROSRJZQVSX-AKYJZCGHSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50073752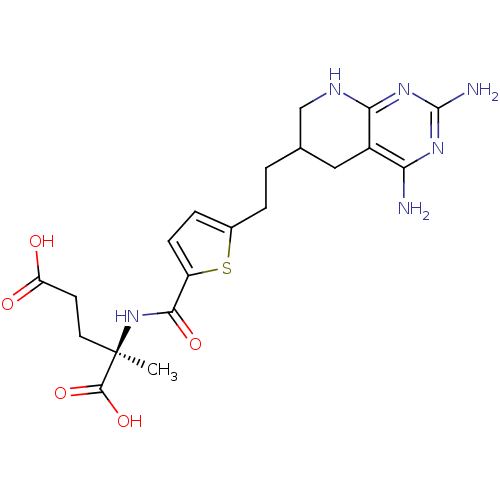 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50073752 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Thymidylate synthase enzyme | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GAR transformylase (Mus musculus) | BDBM50073752 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||