

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50078324 CHEMBL3414708::US9260455, 8
SMILES: [H][C@]12CSC(N)=N[C@]1(CO[C@@H](COC)C2)c1ccc(F)cc1F
InChI Key: InChIKey=AVYXEHSYNNACNZ-ISOBSLSZSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (BACE1) (Homo sapiens (Human)) | BDBM50078324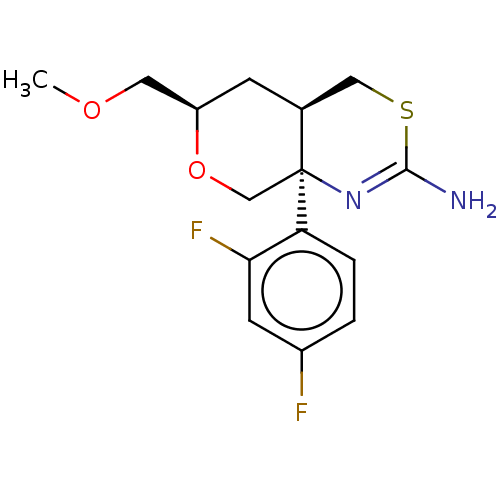 (CHEMBL3414708 | US9260455, 8) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 855 | n/a | n/a | n/a | n/a | 4.5 | 37 |
Pfizer Inc. US Patent | Assay Description A synthetic APP substrate that can be cleaved by beta-secretase having N-terminal biotin and made fluorescent by the covalent attachment of Oregon Gr... | US Patent US9260455 (2016) BindingDB Entry DOI: 10.7270/Q2GB22WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50078324 (CHEMBL3414708 | US9260455, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | 4.5 | 37 |
Pfizer Inc. US Patent | Assay Description This assay measures the inhibition of the BACE2 enzyme as it cleaves a non-native peptide. A synthetic substrate that can be cleaved by BACE2 having ... | US Patent US9260455 (2016) BindingDB Entry DOI: 10.7270/Q2GB22WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078324 (CHEMBL3414708 | US9260455, 8) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50078324 (CHEMBL3414708 | US9260455, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >9.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CatD (unknown origin) by fluorescence polarization assay | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078324 (CHEMBL3414708 | US9260455, 8) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078324 (CHEMBL3414708 | US9260455, 8) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||