

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM210888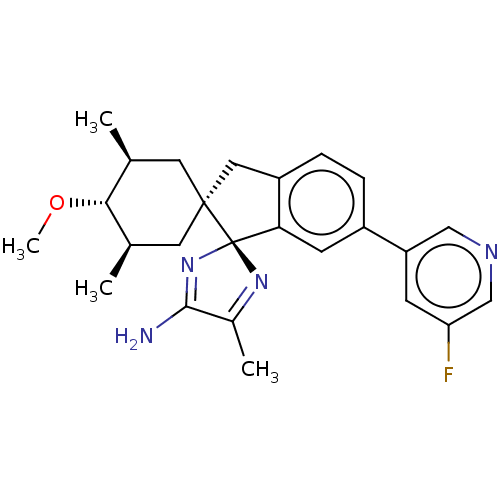 (US9290477, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description The assay was performed in the presence of OptiMEM (supernatant collected over 24 h and cleared from cellular debris by centrifugation) containing th... | US Patent US9290477 (2016) BindingDB Entry DOI: 10.7270/Q2C82853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM210887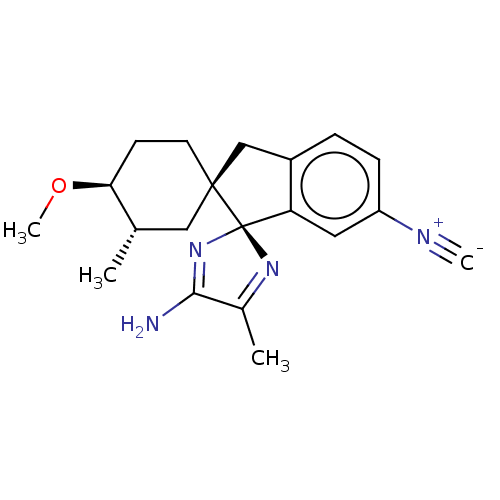 (US9290477, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description The assay was performed in the presence of OptiMEM (supernatant collected over 24 h and cleared from cellular debris by centrifugation) containing th... | US Patent US9290477 (2016) BindingDB Entry DOI: 10.7270/Q2C82853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150700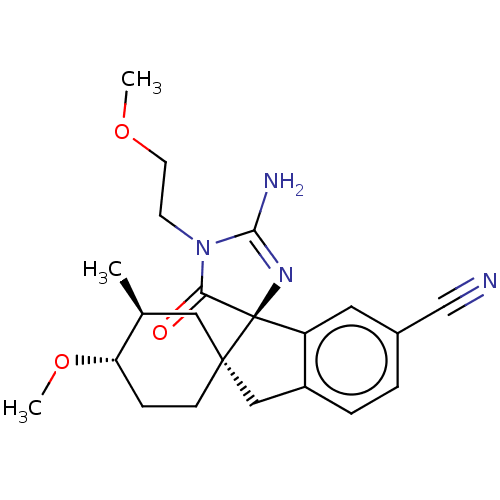 (US8981112, 7 | US9526727, 7 | US9949975, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150705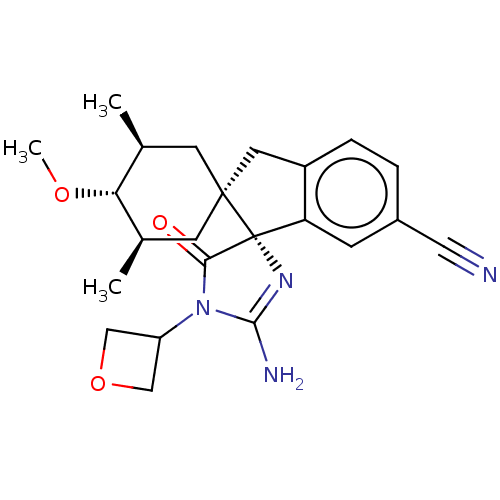 (US8981112, 12 | US9526727, 12 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM210884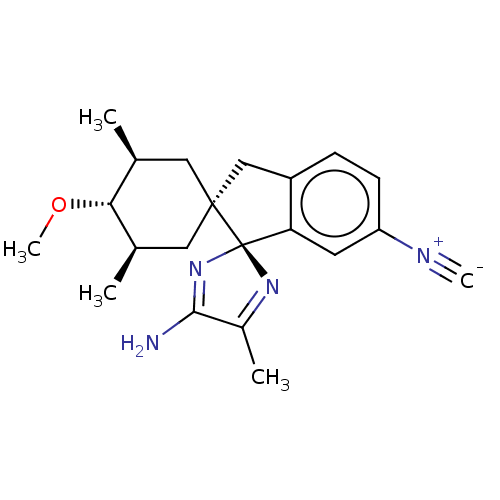 (US9290477, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description The assay was performed in the presence of OptiMEM (supernatant collected over 24 h and cleared from cellular debris by centrifugation) containing th... | US Patent US9290477 (2016) BindingDB Entry DOI: 10.7270/Q2C82853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM210883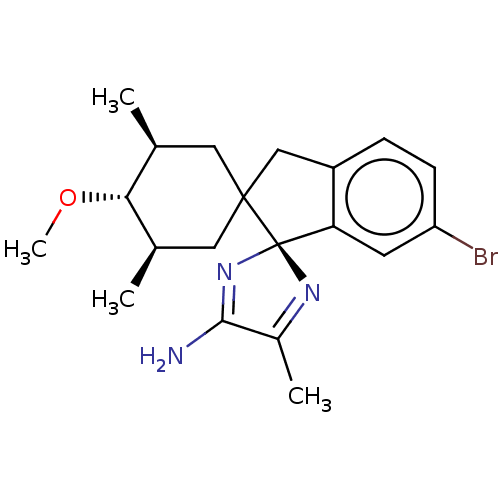 (US9290477, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description The assay was performed in the presence of OptiMEM (supernatant collected over 24 h and cleared from cellular debris by centrifugation) containing th... | US Patent US9290477 (2016) BindingDB Entry DOI: 10.7270/Q2C82853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150704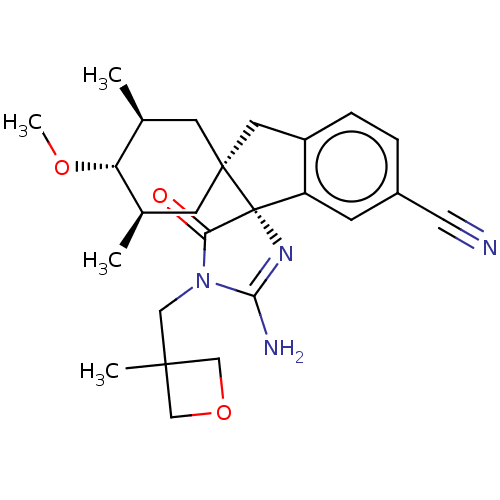 (US8981112, 11 | US9526727, 11 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150707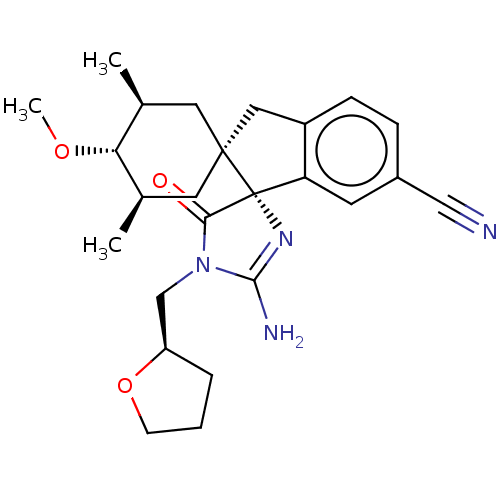 (US8981112, 14 | US9526727, 14 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150701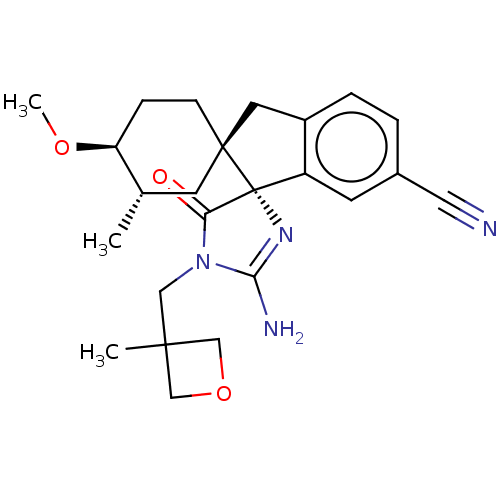 (US8981112, 8 | US9526727, 8 | US9949975, Example 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150697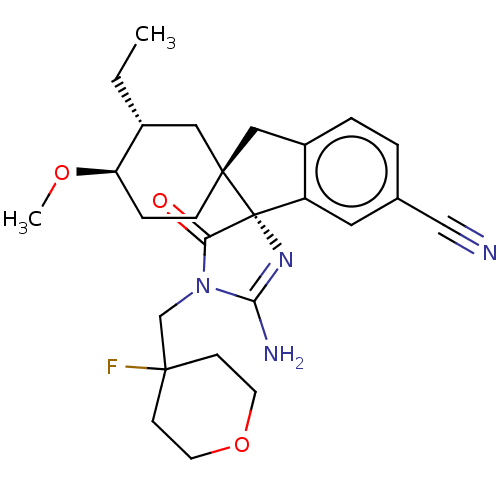 (US8981112, 4 | US9526727, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150717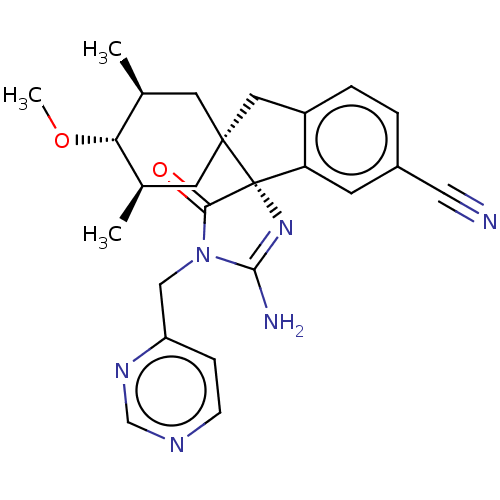 (US8981112, 24 | US9526727, 24 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150714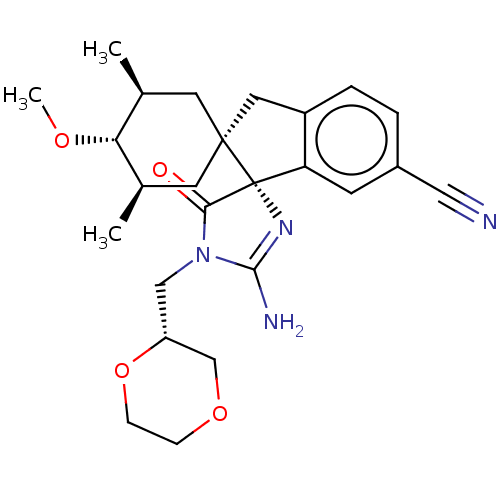 (US8981112, 21 | US9526727, 21 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150712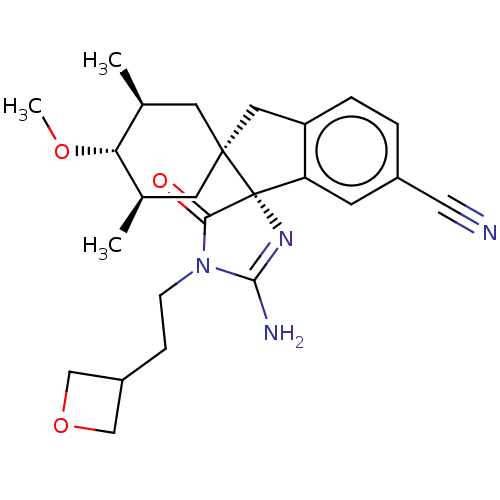 (US8981112, 19 | US9526727, 19 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150720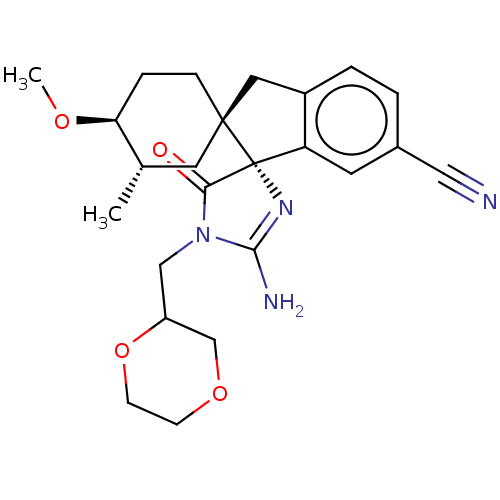 (US8981112, 27 | US9526727, 27 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150715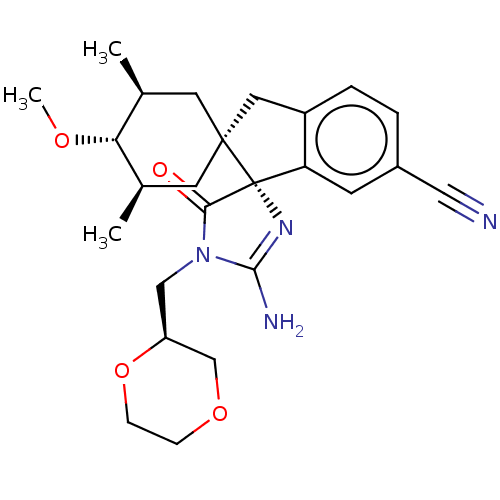 (US8981112, 22 | US9526727, 22 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM259489 (US9526727, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150695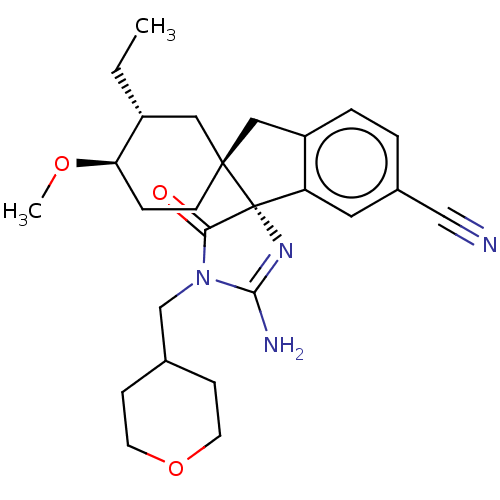 (US8981112, 2 | US9526727, 2 | US9949975, Example 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM210889 (US9290477, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description The assay was performed in the presence of OptiMEM (supernatant collected over 24 h and cleared from cellular debris by centrifugation) containing th... | US Patent US9290477 (2016) BindingDB Entry DOI: 10.7270/Q2C82853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150706 (US8981112, 13 | US9526727, 13 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150719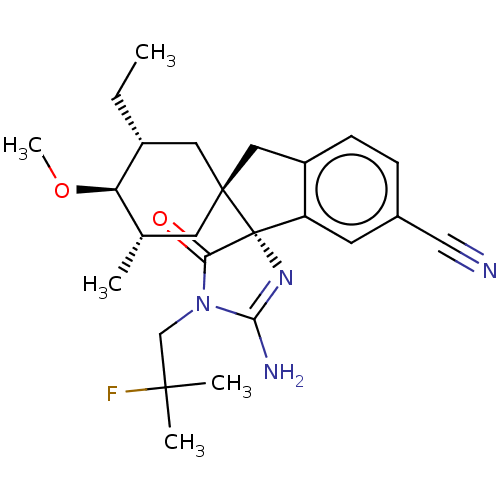 (US8981112, 26 | US9526727, 26 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150703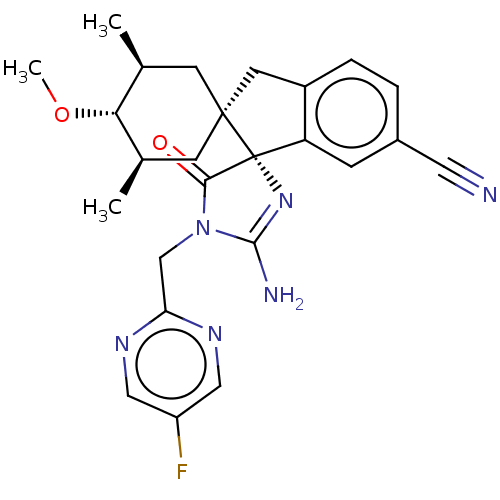 (US8981112, 10 | US9526727, 10 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150711 (US8981112, 18 | US9526727, 18 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150702 (US8981112, 9 | US9526727, 9 | US9949975, Example 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150696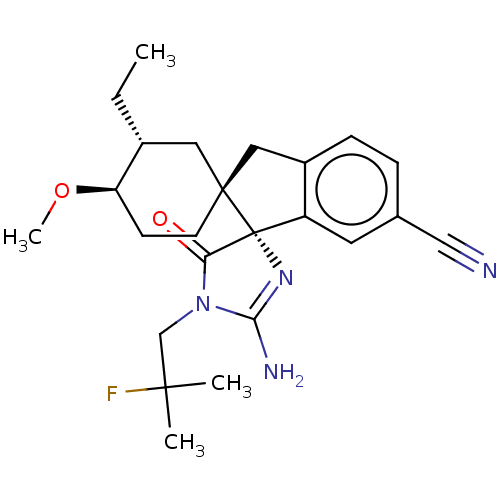 (US8981112, 3 | US9526727, 3 | US9949975, Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150709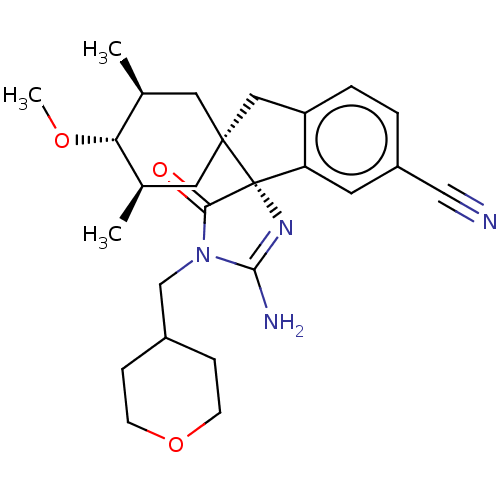 (US8981112, 16 | US9526727, 16 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150699 (US8981112, 6 | US9526727, 6 | US9949975, Example 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM259488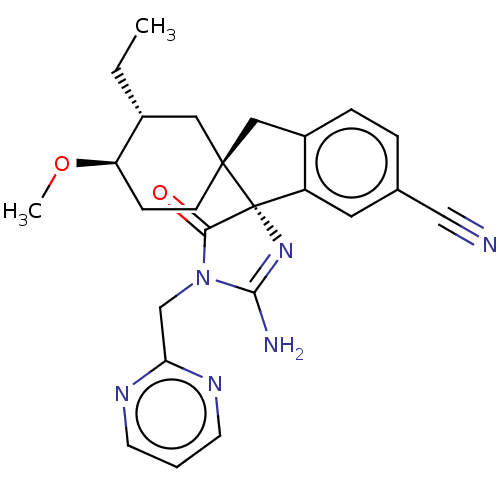 (US9526727, 1 | US9949975, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150708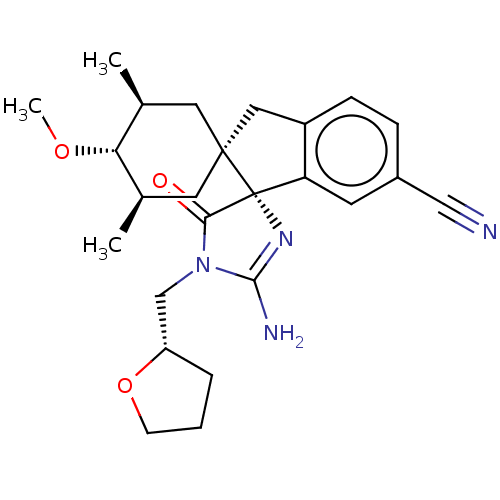 (US8981112, 15 | US9526727, 15 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150716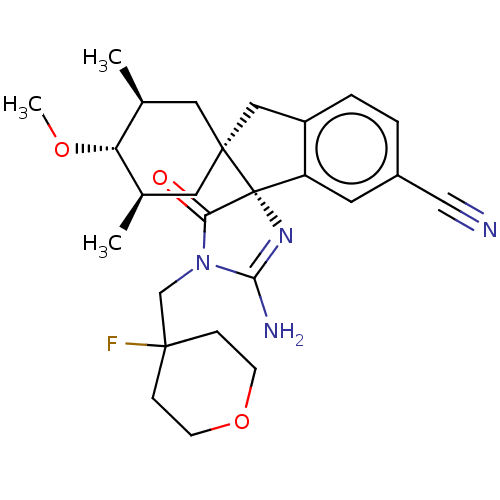 (US8981112, 23 | US9526727, 23 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM150710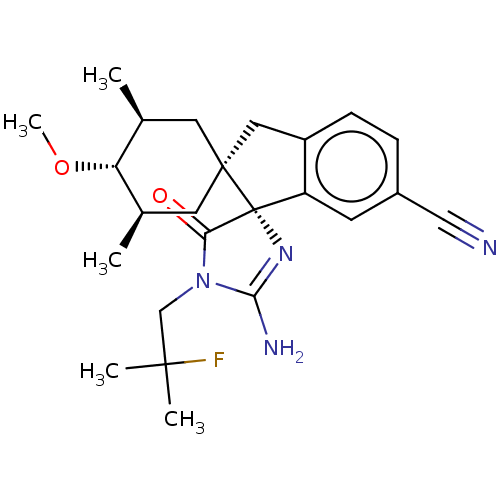 (US8981112, 17 | US9526727, 17 | US9949975, Example...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM210886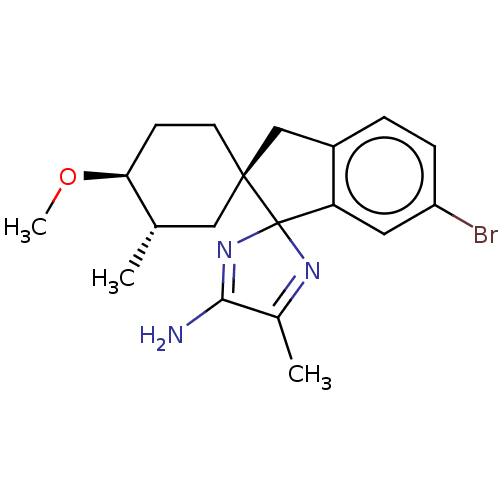 (US9290477, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.3 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description The assay was performed in the presence of OptiMEM (supernatant collected over 24 h and cleared from cellular debris by centrifugation) containing th... | US Patent US9290477 (2016) BindingDB Entry DOI: 10.7270/Q2C82853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM259485 (US9526727, Comparison Example Table 2, 2''-amino-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM210885 (US9290477, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description The assay was performed in the presence of OptiMEM (supernatant collected over 24 h and cleared from cellular debris by centrifugation) containing th... | US Patent US9290477 (2016) BindingDB Entry DOI: 10.7270/Q2C82853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM259486 (US9526727, Comparison Example Table 2, (1r,4r)-2''...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM195494 (US9212153, 158,Ex. 121 | US9526727, Comparison Exa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 107 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-454] (Homo sapiens (Human)) | BDBM259487 (US9526727, Comparison Example Table 2, (1r,1'R,4R)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 415 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceutical, Inc. US Patent | Assay Description The inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE1 activity using commercially available substrate HiLyte Fluo... | US Patent US9526727 (2016) BindingDB Entry DOI: 10.7270/Q2K936GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||