

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50093745 (6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-methyl-1H-tetrazol-5-ylsulfanylmethyl)-8-oxo-5-oxa-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid 4-carboxy-benzyl ester::CHEMBL295221
SMILES: CCOc1ccccc1C(=O)N[C@@]1(OC)[C@H]2OCC(CSc3nnnn3C)=C(N2C1=O)C(=O)OCc1ccc(cc1)C(O)=O
InChI Key: InChIKey=VUEVZBHUKRORBJ-IAPPQJPRSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50093745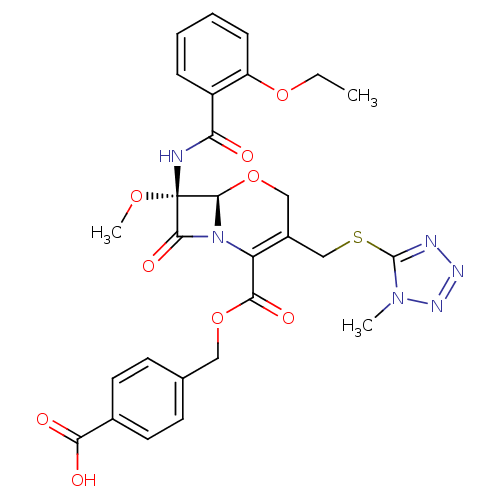 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against alpha-chymotrypsin | Bioorg Med Chem Lett 10: 2403-6 (2001) BindingDB Entry DOI: 10.7270/Q23R0S4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50093745 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human Serine protease chymase | Bioorg Med Chem Lett 10: 2403-6 (2001) BindingDB Entry DOI: 10.7270/Q23R0S4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093745 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against trypsin | Bioorg Med Chem Lett 10: 2403-6 (2001) BindingDB Entry DOI: 10.7270/Q23R0S4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50093745 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human Serine protease chymase | Bioorg Med Chem Lett 11: 1695-7 (2001) BindingDB Entry DOI: 10.7270/Q2Q81CB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50093745 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against plasmin | Bioorg Med Chem Lett 10: 2403-6 (2001) BindingDB Entry DOI: 10.7270/Q23R0S4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093745 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against thrombin | Bioorg Med Chem Lett 10: 2403-6 (2001) BindingDB Entry DOI: 10.7270/Q23R0S4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50093745 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against cathepsin G | Bioorg Med Chem Lett 10: 2403-6 (2001) BindingDB Entry DOI: 10.7270/Q23R0S4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50093745 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against elastase | Bioorg Med Chem Lett 10: 2403-6 (2001) BindingDB Entry DOI: 10.7270/Q23R0S4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||