

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50100341 (2E,4E,6E,12E)-(8R,10S,14R)-8,10,12,14-Tetramethyl-hexadeca-2,4,6,12-tetraenoic acid [(S)-1-hydroxymethyl-2-((1S,2S,6S)-2-hydroxy-3-oxo-7-oxa-bicyclo[4.1.0]hept-4-en-2-yl)-ethyl]-amide::CHEMBL418376
SMILES: CC[C@@H](C)\C=C(/C)C[C@@H](C)C[C@@H](C)\C=C\C=C\C=C\C(=O)N[C@H](CO)C[C@]1(O)[C@H]2O[C@H]2C=CC1=O
InChI Key: InChIKey=KSIWZCYBCSQXTA-JPOQAJQISA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral sphingomyelinase (Rattus norvegicus) | BDBM50100341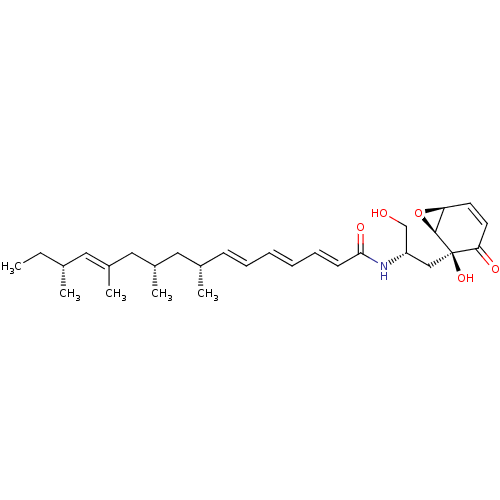 ((2E,4E,6E,12E)-(8R,10S,14R)-8,10,12,14-Tetramethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory concentration against neutral sphingomyelinase (N-Smase) from rat brain microsomes | Bioorg Med Chem Lett 11: 1277-80 (2001) BindingDB Entry DOI: 10.7270/Q2B56J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral sphingomyelinase (Rattus norvegicus) | BDBM50100341 ((2E,4E,6E,12E)-(8R,10S,14R)-8,10,12,14-Tetramethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against the membrane neutral magnesium-dependent Sphingomyelinase (N-SMase) using rat brain microsomes as the enzyme source | Bioorg Med Chem Lett 13: 1963-6 (2003) BindingDB Entry DOI: 10.7270/Q2KD1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral sphingomyelinase (Homo sapiens (Human)) | BDBM50100341 ((2E,4E,6E,12E)-(8R,10S,14R)-8,10,12,14-Tetramethyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral sphingomyelinase (Rattus norvegicus) | BDBM50100341 ((2E,4E,6E,12E)-(8R,10S,14R)-8,10,12,14-Tetramethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory concentration against lysosomal neutral sphingomyelinase (N-Smase) | Bioorg Med Chem Lett 11: 1277-80 (2001) BindingDB Entry DOI: 10.7270/Q2B56J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||