

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50108306 (S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine::(S)-2-(5,6-Difluoro-indol-1-yl)-1-methyl-ethylamine::CHEMBL40726
SMILES: C[C@H](N)Cn1ccc2cc(F)c(F)cc12
InChI Key: InChIKey=MYZDBEVPJGKIQS-ZETCQYMHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50108306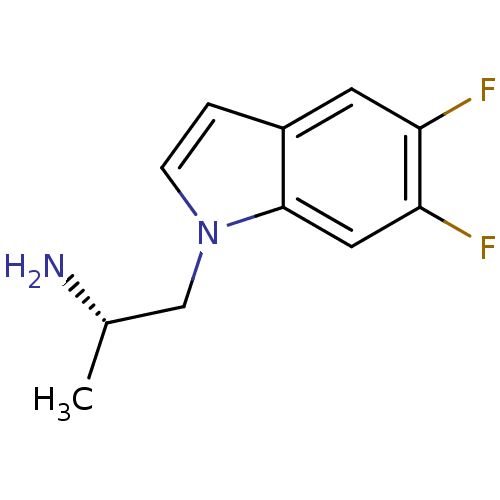 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Health Science Center Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human cloned 5HT2C receptor expressed in HEK293 cells after 90 mins by liquid scintillation counting | Bioorg Med Chem 18: 4783-92 (2011) Article DOI: 10.1016/j.bmc.2010.05.017 BindingDB Entry DOI: 10.7270/Q2V69KJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50108306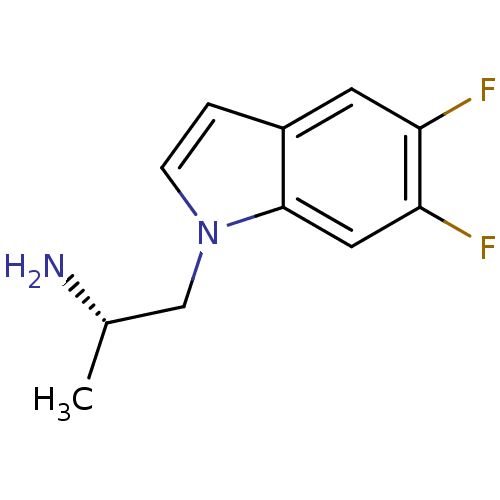 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50108306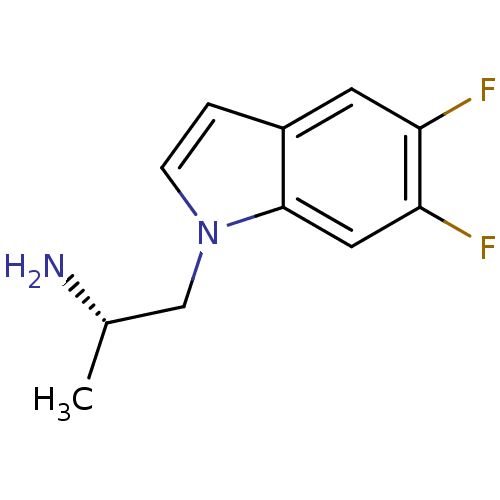 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Tested for binding affinity of the compound towards human 5-hydroxytryptamine 2C receptor using [3H]-DOB as radioligand | Bioorg Med Chem Lett 12: 155-8 (2001) BindingDB Entry DOI: 10.7270/Q21G0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50108306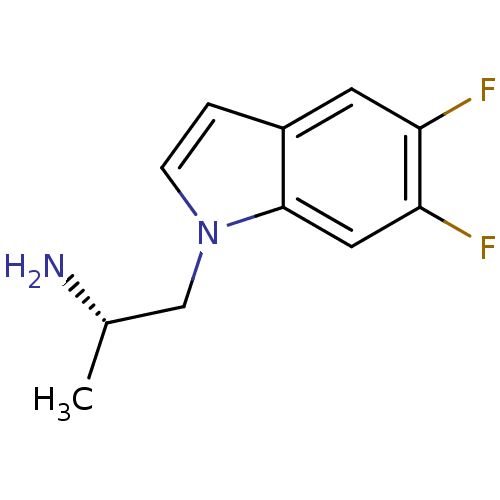 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-DOB from human 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 12: 155-8 (2001) BindingDB Entry DOI: 10.7270/Q21G0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108306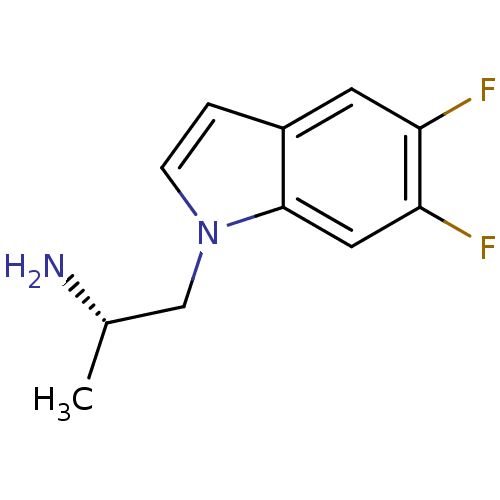 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Tested for binding affinity of the compound towards rat 5-hydroxytryptamine 2A receptor using [3H]-Ketanserin as radioligand | Bioorg Med Chem Lett 12: 155-8 (2001) BindingDB Entry DOI: 10.7270/Q21G0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108306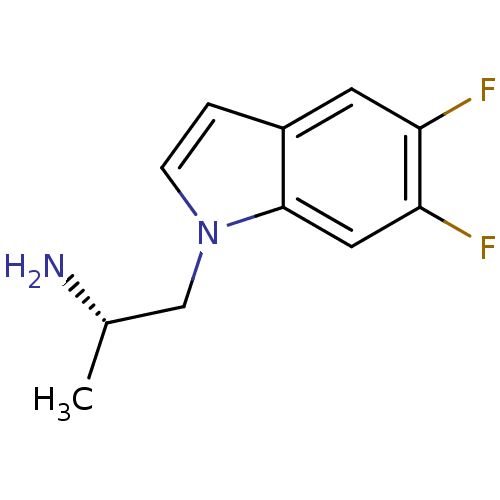 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Tested for binding affinity of the compound towards rat 5-hydroxytryptamine 2A receptor using [3H]-DOB as radioligand | Bioorg Med Chem Lett 12: 155-8 (2001) BindingDB Entry DOI: 10.7270/Q21G0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108306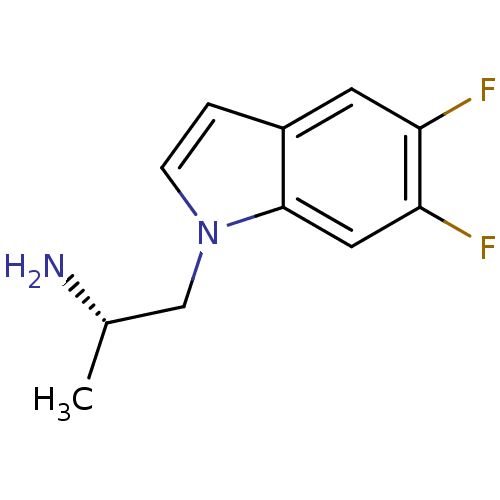 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Tested for binding affinity of the compound towards human 5-hydroxytryptamine 2A receptor using [3H]-DOB as radioligand | Bioorg Med Chem Lett 12: 155-8 (2001) BindingDB Entry DOI: 10.7270/Q21G0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50108306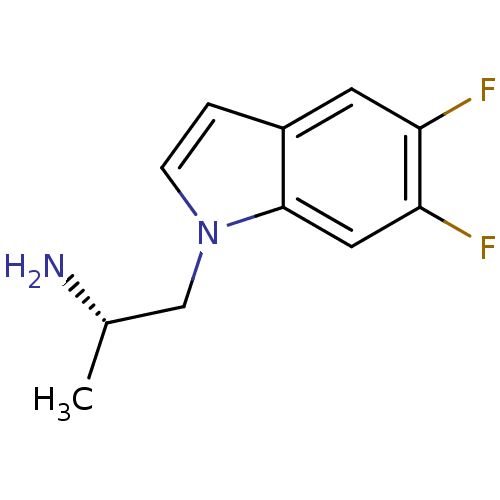 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Tested for binding affinity of the compound towards human 5-hydroxytryptamine 2C receptor using [3H]-5-HT as radioligand | Bioorg Med Chem Lett 12: 155-8 (2001) BindingDB Entry DOI: 10.7270/Q21G0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50108306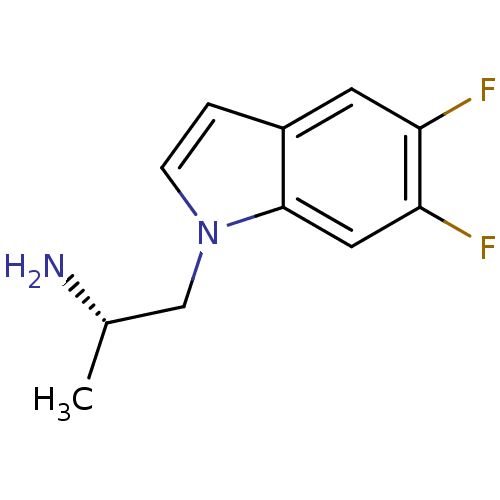 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Tested for binding affinity of the compound towards human 5-hydroxytryptamine 2C receptor using [3H]-Mesulergine as radioligand | Bioorg Med Chem Lett 12: 155-8 (2001) BindingDB Entry DOI: 10.7270/Q21G0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108306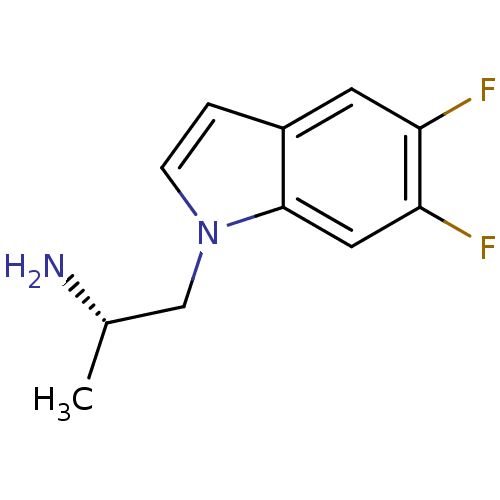 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Health Science Center Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human cloned 5HT2A receptor expressed in HEK293 cells after 90 mins by liquid scintillation counting | Bioorg Med Chem 18: 4783-92 (2011) Article DOI: 10.1016/j.bmc.2010.05.017 BindingDB Entry DOI: 10.7270/Q2V69KJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108306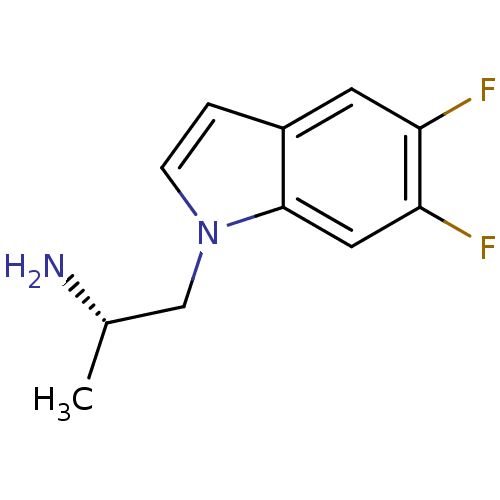 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2A receptor using displacement of [3H]5-HT | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108306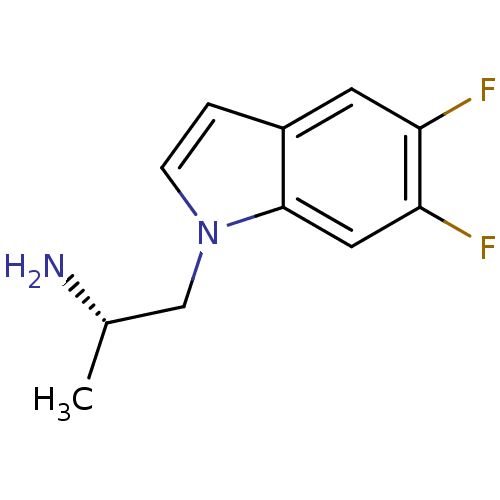 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-DOB from human 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 12: 155-8 (2001) BindingDB Entry DOI: 10.7270/Q21G0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50108306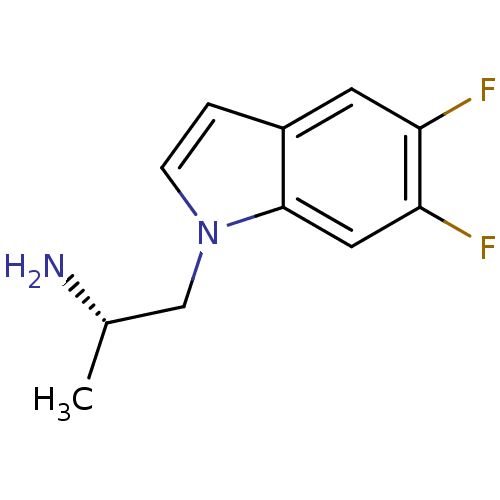 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Efficacy (pEC50) was evaluated for 5-HT2C receptor-mediated stimulation of IP3 formation in vitro in choroid plexus of the rat | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||