

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50111438 3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methyl}-4-(2-imidazol-1-yl-pyrimidin-4-yl)-piperazine-1-carboxylic acid methyl ester::CHEMBL290548::N-[(1,3-benzodioxol-5-yl)methyl]-1-[2-(1H-imidazol-1-yl)pyrimidin-4-yl]-4-[(methoxy)carbonyl]-2-piperazineacetamide
SMILES: COC(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1
InChI Key: InChIKey=NVYMEDQKBQMAKF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50111438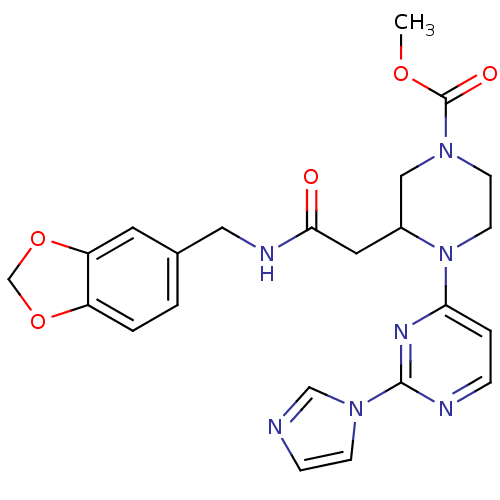 (3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding against the partially purified human Inducible nitric oxide synthase | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50111438 (3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against the partially purified human Inducible nitric oxide synthase | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50111438 (3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation | J Med Chem 50: 1146-57 (2007) Article DOI: 10.1021/jm061319i BindingDB Entry DOI: 10.7270/Q2SX6CWJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||