

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: OP(O)(=O)C(=O)NCCc1ccccc1
InChI Key: InChIKey=GHFCXEYUGIXTHN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50112446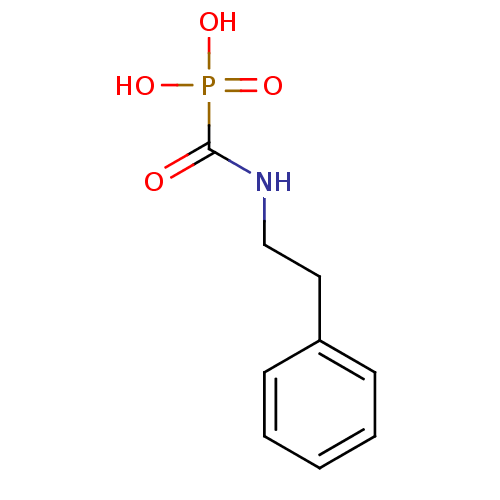 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards phosphotyrosine binding pocket of Src protein tryrosine kinase SH2 domain | Bioorg Med Chem Lett 12: 1295-8 (2002) BindingDB Entry DOI: 10.7270/Q2959GW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant matrix metalloprotease-3 was determined | J Med Chem 47: 2826-32 (2004) Article DOI: 10.1021/jm030386z BindingDB Entry DOI: 10.7270/Q2XS5W4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant matrix metalloprotease-8 was determined | J Med Chem 47: 2826-32 (2004) Article DOI: 10.1021/jm030386z BindingDB Entry DOI: 10.7270/Q2XS5W4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant matrix metalloprotease-9 was determined | J Med Chem 47: 2826-32 (2004) Article DOI: 10.1021/jm030386z BindingDB Entry DOI: 10.7270/Q2XS5W4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant matrix metalloprotease-2 was determined | J Med Chem 47: 2826-32 (2004) Article DOI: 10.1021/jm030386z BindingDB Entry DOI: 10.7270/Q2XS5W4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Inhibition of MMP2 | J Med Chem 55: 7875-82 (2012) Article DOI: 10.1021/jm300981b BindingDB Entry DOI: 10.7270/Q23T9JBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Inhibition of human Carbonic anhydrase 12-mediated CO2 hydration by stopped-flow method | J Med Chem 55: 7875-82 (2012) Article DOI: 10.1021/jm300981b BindingDB Entry DOI: 10.7270/Q23T9JBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Inhibition of human Carbonic anhydrase 9-mediated CO2 hydration by stopped-flow method | J Med Chem 55: 7875-82 (2012) Article DOI: 10.1021/jm300981b BindingDB Entry DOI: 10.7270/Q23T9JBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Inhibition of human Carbonic anhydrase 2-mediated CO2 hydration by stopped-flow method | J Med Chem 55: 7875-82 (2012) Article DOI: 10.1021/jm300981b BindingDB Entry DOI: 10.7270/Q23T9JBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Inhibition of human Carbonic anhydrase 1-mediated CO2 hydration by stopped-flow method | J Med Chem 55: 7875-82 (2012) Article DOI: 10.1021/jm300981b BindingDB Entry DOI: 10.7270/Q23T9JBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50112446 (CHEMBL24447 | [(2-phenylethyl)amino]carbonylphosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant matrix metalloprotease-1 was determined | J Med Chem 47: 2826-32 (2004) Article DOI: 10.1021/jm030386z BindingDB Entry DOI: 10.7270/Q2XS5W4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||