

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50127375 (E)-(R)-4-Hydroxy-2-methyl-hex-2-enedioic acid 6-{(1R,2S,3R,6S,7S,10R)-10-[(2S,3S,6R,8S,9R)-3,9-dimethyl-8-((S)-3-methyl-4-oxo-pentyl)-1,7-dioxa-spiro[5.5]undec-2-yl]-3,7-dihydroxy-1-isopropyl-2-methoxy-6-methyl-5-oxo-undecyl} ester::CHEMBL295239
SMILES: CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)\C=C(/C)C(O)=O)C(C)C
InChI Key: InChIKey=GWMVRWABGZUAHZ-YSOIJUGWSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein phosphatase 2A regulatory subunit B' (Homo sapiens (Human)) | BDBM50127375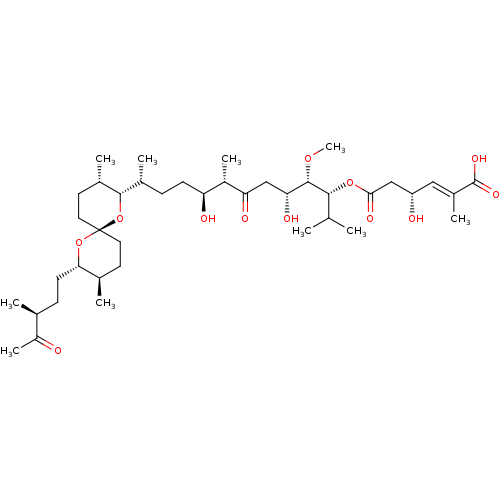 ((E)-(R)-4-Hydroxy-2-methyl-hex-2-enedioic acid 6-{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay | Bioorg Med Chem Lett 13: 1597-600 (2003) BindingDB Entry DOI: 10.7270/Q2DB82CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||