

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50136798 CHEMBL410372::Somatostatin analogue
SMILES: C[C@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)C(O)=O)NC(=O)[C@H](N)Cc1ccc(O)cc1
InChI Key: InChIKey=LWKOEELVPGZRCE-HSUQGJCQSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50136798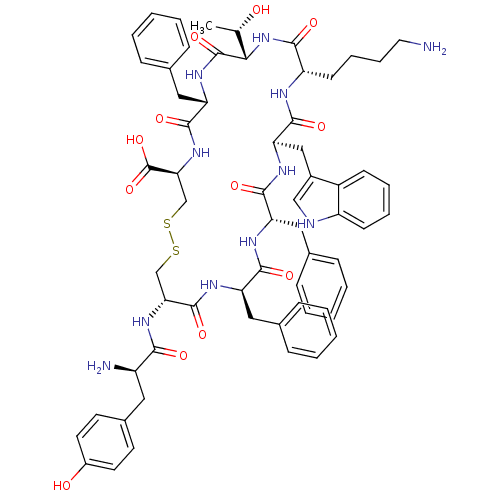 (CHEMBL410372 | Somatostatin analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Binding affinity towards human somatostatin receptor type 1 using [125I]-[Leu8,DTrp22,Tyr25]SRIF-28 as radioligand. | J Med Chem 46: 5597-605 (2003) Article DOI: 10.1021/jm030245x BindingDB Entry DOI: 10.7270/Q2MK6C9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50136798 (CHEMBL410372 | Somatostatin analogue) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Binding affinity towards human somatostatin receptor type 3 using [125I]-[Leu8,DTrp22,Tyr25]SRIF-28 as radioligand. | J Med Chem 46: 5597-605 (2003) Article DOI: 10.1021/jm030245x BindingDB Entry DOI: 10.7270/Q2MK6C9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50136798 (CHEMBL410372 | Somatostatin analogue) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Binding affinity towards human somatostatin receptor type 4 using [125I]-[Leu8,DTrp22,Tyr25]SRIF-28 as radioligand. | J Med Chem 46: 5597-605 (2003) Article DOI: 10.1021/jm030245x BindingDB Entry DOI: 10.7270/Q2MK6C9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50136798 (CHEMBL410372 | Somatostatin analogue) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Binding affinity towards human somatostatin receptor type 5 using [125I]-[Leu8,DTrp22,Tyr25]SRIF-28 as radioligand. | J Med Chem 46: 5597-605 (2003) Article DOI: 10.1021/jm030245x BindingDB Entry DOI: 10.7270/Q2MK6C9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50136798 (CHEMBL410372 | Somatostatin analogue) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Binding affinity towards human somatostatin receptor type 2 using [125I]-[Leu8,DTrp22,Tyr25]SRIF-28 as radioligand. | J Med Chem 46: 5597-605 (2003) Article DOI: 10.1021/jm030245x BindingDB Entry DOI: 10.7270/Q2MK6C9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||