 Found 6 hits for monomerid = 50139187
Found 6 hits for monomerid = 50139187 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Geranylgeranyl transferase type I beta subunit/Protein Farnesyltransferase (PFT)
(Homo sapiens (Human)) | BDBM50139187
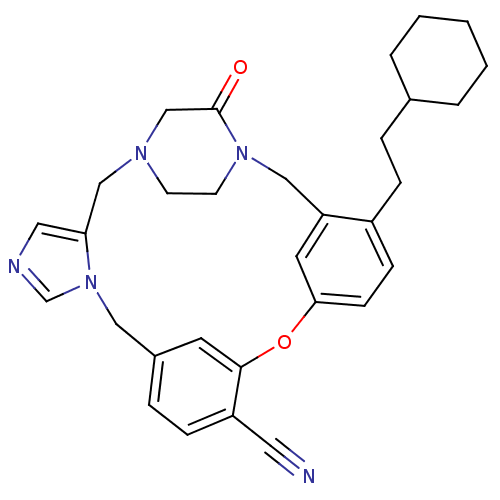
(4-(2-cyclohexylethyl)-23-oxo-8-oxa-1,15,17,21-tetr...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CCC1CCCCC1 Show InChI InChI=1S/C31H35N5O2/c32-16-26-9-7-24-14-30(26)38-29-11-10-25(8-6-23-4-2-1-3-5-23)27(15-29)19-35-13-12-34(21-31(35)37)20-28-17-33-22-36(28)18-24/h7,9-11,14-15,17,22-23H,1-6,8,12-13,18-21H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Geranylgeranyl transferase type I incorporation of [3H]-GGPP into biotinylated peptide corresponding to the C-terminus of human K... |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein Farnesyltransferase (PFT)
(Homo sapiens (Human)) | BDBM50139187

(4-(2-cyclohexylethyl)-23-oxo-8-oxa-1,15,17,21-tetr...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CCC1CCCCC1 Show InChI InChI=1S/C31H35N5O2/c32-16-26-9-7-24-14-30(26)38-29-11-10-25(8-6-23-4-2-1-3-5-23)27(15-29)19-35-13-12-34(21-31(35)37)20-28-17-33-22-36(28)18-24/h7,9-11,14-15,17,22-23H,1-6,8,12-13,18-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
DnaJ homolog subfamily A member 1
(Homo sapiens (Human)) | BDBM50139187

(4-(2-cyclohexylethyl)-23-oxo-8-oxa-1,15,17,21-tetr...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CCC1CCCCC1 Show InChI InChI=1S/C31H35N5O2/c32-16-26-9-7-24-14-30(26)38-29-11-10-25(8-6-23-4-2-1-3-5-23)27(15-29)19-35-13-12-34(21-31(35)37)20-28-17-33-22-36(28)18-24/h7,9-11,14-15,17,22-23H,1-6,8,12-13,18-21H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of HDJ2 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50139187

(4-(2-cyclohexylethyl)-23-oxo-8-oxa-1,15,17,21-tetr...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CCC1CCCCC1 Show InChI InChI=1S/C31H35N5O2/c32-16-26-9-7-24-14-30(26)38-29-11-10-25(8-6-23-4-2-1-3-5-23)27(15-29)19-35-13-12-34(21-31(35)37)20-28-17-33-22-36(28)18-24/h7,9-11,14-15,17,22-23H,1-6,8,12-13,18-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of Ki-Ras |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Ras-related protein Rap-1A
(Homo sapiens (Human)) | BDBM50139187

(4-(2-cyclohexylethyl)-23-oxo-8-oxa-1,15,17,21-tetr...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CCC1CCCCC1 Show InChI InChI=1S/C31H35N5O2/c32-16-26-9-7-24-14-30(26)38-29-11-10-25(8-6-23-4-2-1-3-5-23)27(15-29)19-35-13-12-34(21-31(35)37)20-28-17-33-22-36(28)18-24/h7,9-11,14-15,17,22-23H,1-6,8,12-13,18-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of Rap1a |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Protein Farnesyltransferase (PFT)
(Homo sapiens (Human)) | BDBM50139187

(4-(2-cyclohexylethyl)-23-oxo-8-oxa-1,15,17,21-tetr...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CCC1CCCCC1 Show InChI InChI=1S/C31H35N5O2/c32-16-26-9-7-24-14-30(26)38-29-11-10-25(8-6-23-4-2-1-3-5-23)27(15-29)19-35-13-12-34(21-31(35)37)20-28-17-33-22-36(28)18-24/h7,9-11,14-15,17,22-23H,1-6,8,12-13,18-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data