

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50142692 CHEMBL3759092
SMILES: [H][C@]12Cc3ccc(O)cc3[C@](C)(CCN1CCC(=O)Nc1ccccc1)[C@H]2C
InChI Key: InChIKey=VGNUFLUEYYNYCK-POCHDABASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50142692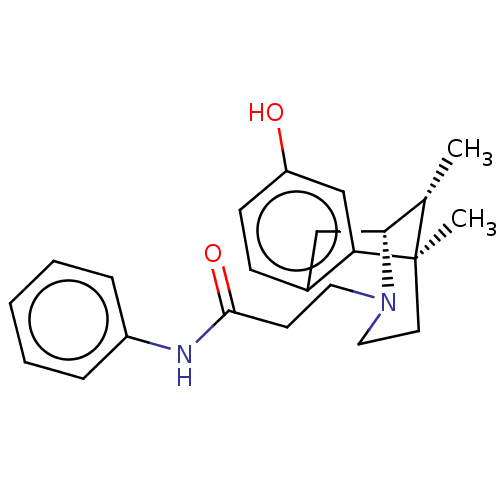 (CHEMBL3759092) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Binding affinity at mu opioid receptor (unknown origin) | Eur J Med Chem 108: 211-28 (2016) BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Drug Sciences, Medicinal Chemistry Section, University of Catania, Viale A. Doria 6, 95125 Catania, Italy. Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from MOR in rat brain membranes by liquid scintillation counting analysis | Bioorg Med Chem 25: 4745-4752 (2017) Article DOI: 10.1016/j.bmc.2017.07.021 BindingDB Entry DOI: 10.7270/Q2S46VG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50142692 (CHEMBL3759092) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in HEK293 cells | Bioorg Med Chem 24: 5280-5290 (2016) Article DOI: 10.1016/j.bmc.2016.08.057 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Binding affinity at delta opioid receptor (unknown origin) | Eur J Med Chem 108: 211-28 (2016) BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Drug Sciences, Medicinal Chemistry Section, University of Catania, Viale A. Doria 6, 95125 Catania, Italy. Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from DOR in rat brain membranes by liquid scintillation counting analysis | Bioorg Med Chem 25: 4745-4752 (2017) Article DOI: 10.1016/j.bmc.2017.07.021 BindingDB Entry DOI: 10.7270/Q2S46VG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human DOR expressed in CHO cells | Bioorg Med Chem 24: 5280-5290 (2016) Article DOI: 10.1016/j.bmc.2016.08.057 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-DPN from rat DOR expressed in EE-HEK293 cell membranes by liquid scintillation counting | Bioorg Med Chem 24: 2832-42 (2016) BindingDB Entry DOI: 10.7270/Q2VH5QR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-DPN from rat MOR expressed in EE-HEK293 cell membranes by liquid scintillation counting | Bioorg Med Chem 24: 2832-42 (2016) BindingDB Entry DOI: 10.7270/Q2VH5QR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50142692 (CHEMBL3759092) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Drug Sciences, Medicinal Chemistry Section, University of Catania, Viale A. Doria 6, 95125 Catania, Italy. Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-U69,593 from KOR in guinea pig brain membranes by liquid scintillation counting analysis | Bioorg Med Chem 25: 4745-4752 (2017) Article DOI: 10.1016/j.bmc.2017.07.021 BindingDB Entry DOI: 10.7270/Q2S46VG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor (Rattus norvegicus (rat)) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from rat KOR expressed in CHO cells | Bioorg Med Chem 24: 5280-5290 (2016) Article DOI: 10.1016/j.bmc.2016.08.057 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Activity at human KOR expressed in EE-HEK293 cells assessed as pEC50 for effect on (-)U50,488H-induced response (Rvb = 6.2 +/- 0.04 No_unit) | Bioorg Med Chem 24: 2832-42 (2016) BindingDB Entry DOI: 10.7270/Q2VH5QR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| mu/kappa opioid receptor (Cavia porcellus (domestic guinea pig)-GUINEA PIG) | BDBM50142692 (CHEMBL3759092) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Drug Sciences, Medicinal Chemistry Section, University of Catania, Viale A. Doria 6, 95125 Catania, Italy. Curated by ChEMBL | Assay Description Agonist activity at MOR/KOR in Dunkin-Hartley guinea pig ileum assessed as inhibition of electrically induced contractions after 5 mins | Bioorg Med Chem 25: 4745-4752 (2017) Article DOI: 10.1016/j.bmc.2017.07.021 BindingDB Entry DOI: 10.7270/Q2S46VG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50142692 (CHEMBL3759092) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Antagonist activity at DOR in CD1 mouse vas deferens assessed as pEC50 for DPDPE-induced inhibition of EFS-evoked contractions at 1 uM incubated for ... | Bioorg Med Chem 24: 2832-42 (2016) BindingDB Entry DOI: 10.7270/Q2VH5QR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50142692 (CHEMBL3759092) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at MOR in Dunkin-Hartley guinea pig ileum assessed as inhibition of EFS-induced contractions incubated for 60 mins | Bioorg Med Chem 24: 2832-42 (2016) BindingDB Entry DOI: 10.7270/Q2VH5QR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||