

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50147998 2-(R)-Cyclohexyl-2-guanidino-N-{2-oxo-2-[4-(5-o-tolyl-2H-pyrazol-3-yl)-piperidin-1-yl]-ethyl}-acetamide::CHEMBL104791
SMILES: Cc1ccccc1-c1cc([nH]n1)C1CCN(CC1)C(=O)CNC(=O)[C@H](N=C(N)N)C1CCCCC1
InChI Key: InChIKey=WOHFHFBHDZCIKD-XMMPIXPASA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interleukin-2 receptor alpha chain (Mus musculus) | BDBM50147998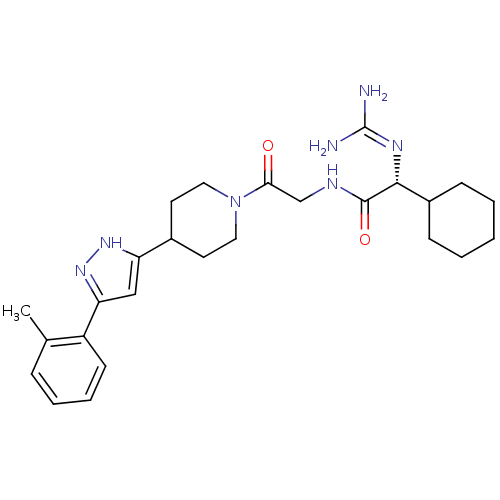 (2-(R)-Cyclohexyl-2-guanidino-N-{2-oxo-2-[4-(5-o-to...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration required to inhibit mouse interleukin-2 alpha receptor was determined by SPA assay | J Med Chem 47: 3111-30 (2004) Article DOI: 10.1021/jm049967u BindingDB Entry DOI: 10.7270/Q2TQ6280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||