

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50199896 3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octahydro-1H-quinolizin-2-yl)phenol::3-((2R,3R,7S,9aS)-2,3-dimethyl-7-phenyl-octahydro-1H-quinolizin-2-yl)phenol::CHEMBL426523::US8580788, 56
SMILES: C[C@H]1CN2C[C@@H](CC[C@H]2C[C@@]1(C)c1cccc(O)c1)c1ccccc1
InChI Key: InChIKey=RGXBTFPNFYCKOY-QYAKYFNYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199896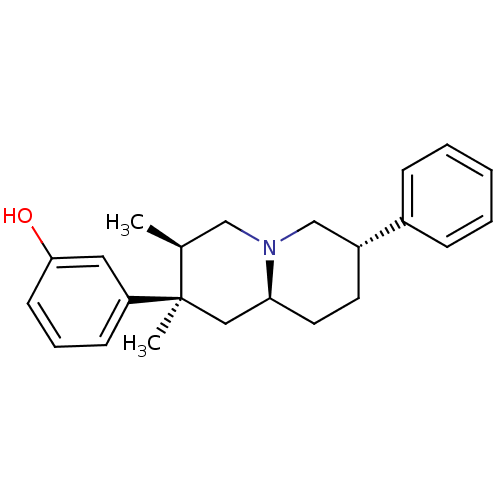 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.570 | -12.6 | 0.530 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Adolor Corporation US Patent | Assay Description The receptor binding method (DeHaven and DeHaven-Hudkins, 1998) was a modification of the method of Raynor et al. (1994). | US Patent US8580788 (2013) BindingDB Entry DOI: 10.7270/Q2N29VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells | J Med Chem 49: 7278-89 (2006) Article DOI: 10.1021/jm060486f BindingDB Entry DOI: 10.7270/Q27P906S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 8.90 | -11.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Adolor Corporation US Patent | Assay Description The receptor binding method (DeHaven and DeHaven-Hudkins, 1998) was a modification of the method of Raynor et al. (1994). | US Patent US8580788 (2013) BindingDB Entry DOI: 10.7270/Q2N29VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned kappa opioid receptor expressed in CHO cells | J Med Chem 49: 7278-89 (2006) Article DOI: 10.1021/jm060486f BindingDB Entry DOI: 10.7270/Q27P906S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of U50488-stimulated [35S]GTP-gamma-S binding to human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 30 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Adolor Corporation US Patent | Assay Description The receptor binding method (DeHaven and DeHaven-Hudkins, 1998) was a modification of the method of Raynor et al. (1994). | US Patent US8580788 (2013) BindingDB Entry DOI: 10.7270/Q2N29VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned delta opioid receptor expressed in CHO cells | J Med Chem 49: 7278-89 (2006) Article DOI: 10.1021/jm060486f BindingDB Entry DOI: 10.7270/Q27P906S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned delta opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of U50488-stimulated [35S]GTP-gamma-S binding to human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity against human cloned mu opioid receptor expressed in CHO cells assessed as inhibition of loperamide-stimulated [35S]GTP-gamma-S b... | J Med Chem 49: 7278-89 (2006) Article DOI: 10.1021/jm060486f BindingDB Entry DOI: 10.7270/Q27P906S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity against human cloned delta opioid receptor expressed in CHO cells assessed as inhibition of loperamide-stimulated [35S]GTP-gamma-... | J Med Chem 49: 7278-89 (2006) Article DOI: 10.1021/jm060486f BindingDB Entry DOI: 10.7270/Q27P906S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of loperamide-stimulated [35S]GTPgammaS binding to human mu opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity against human cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of loperamide-stimulated [35S]GTPgammaS ... | J Med Chem 49: 7278-89 (2006) Article DOI: 10.1021/jm060486f BindingDB Entry DOI: 10.7270/Q27P906S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of BW373U86-stimulated [35S]GTP-gamma-S binding to human delta opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||