

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50201176 CHEMBL3943243
SMILES: COc1ccccc1-c1cn2CCCCC(O)CCCCCCCNc3ncc1c2n3
InChI Key: InChIKey=ILONTPDVQVKFCL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50201176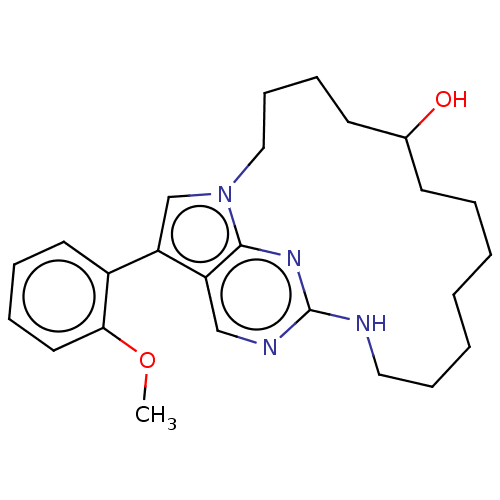 (CHEMBL3943243) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro3 (unknown origin) by microfluidic capillary electrophoresis assay | ACS Med Chem Lett 7: 1044-1049 (2016) Article DOI: 10.1021/acsmedchemlett.6b00221 BindingDB Entry DOI: 10.7270/Q208679K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50201176 (CHEMBL3943243) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Axl (unknown origin) by microfluidic capillary electrophoresis assay | ACS Med Chem Lett 7: 1044-1049 (2016) Article DOI: 10.1021/acsmedchemlett.6b00221 BindingDB Entry DOI: 10.7270/Q208679K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50201176 (CHEMBL3943243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of human MerTK kinase domain (1585 to 3000 residues) expressed in HEK293 cells co-expressing rat EGFR LBD assessed as inhibition of EGF-st... | ACS Med Chem Lett 7: 1044-1049 (2016) Article DOI: 10.1021/acsmedchemlett.6b00221 BindingDB Entry DOI: 10.7270/Q208679K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50201176 (CHEMBL3943243) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Flt3 (unknown origin) by microfluidic capillary electrophoresis assay | ACS Med Chem Lett 7: 1044-1049 (2016) Article DOI: 10.1021/acsmedchemlett.6b00221 BindingDB Entry DOI: 10.7270/Q208679K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50201176 (CHEMBL3943243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay | ACS Med Chem Lett 7: 1044-1049 (2016) Article DOI: 10.1021/acsmedchemlett.6b00221 BindingDB Entry DOI: 10.7270/Q208679K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||