

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50204663 CHEMBL3894489
SMILES: OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCNC(=[Se])Nc1ccc(Br)cc1
InChI Key: InChIKey=JPJTZHGCGLRVGF-YYIAUSFCSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204663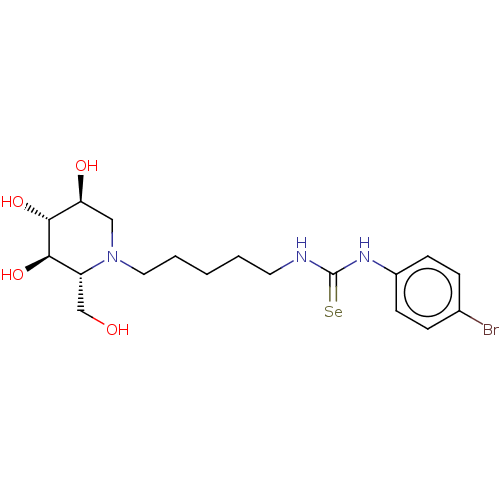 (CHEMBL3894489) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel acetylcholinesterase assessed as enzyme-inhibitor complex by Lineweaver-Burk plot method | Eur J Med Chem 123: 155-160 (2016) Article DOI: 10.1016/j.ejmech.2016.07.021 BindingDB Entry DOI: 10.7270/Q2C24ZD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204663 (CHEMBL3894489) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel acetylcholinesterase assessed as enzyme-substrate-inhibitor complex by Lineweaver-Burk plot method | Eur J Med Chem 123: 155-160 (2016) Article DOI: 10.1016/j.ejmech.2016.07.021 BindingDB Entry DOI: 10.7270/Q2C24ZD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50204663 (CHEMBL3894489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean alpha-mannosidase assessed as enzyme-substrate-inhibitor complex using o-orp-nitrophenyl-glycopyranoside as substr... | Eur J Med Chem 123: 155-160 (2016) Article DOI: 10.1016/j.ejmech.2016.07.021 BindingDB Entry DOI: 10.7270/Q2C24ZD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50204663 (CHEMBL3894489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean alpha-mannosidase assessed as enzyme-inhibitor complex using o-orp-nitrophenyl-glycopyranoside as substrate by Lin... | Eur J Med Chem 123: 155-160 (2016) Article DOI: 10.1016/j.ejmech.2016.07.021 BindingDB Entry DOI: 10.7270/Q2C24ZD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||