

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50227057 (S)-2-((S)-2-((S)-2-((S)-1-((2S,3S)-3-((S)-2-((S)-5-amino-5-oxo-2-((S)-pyrrolidine-2-carboxamido)pentanamido)-3-methylbutanamido)-2-hydroxy-4-phenylbutanoyl)pyrrolidine-2-carboxamido)-3-methylbutanamido)-4-(methylthio)butanamido)-3-(1H-imidazol-5-yl)propanoic acid::(S)-2-((S)-2-((S)-2-((S)-1-((2S,3S)-3-((S)-2-((S)-5-amino-5-oxo-2-((S)-pyrrolidine-2-carboxamido)pentanamido)-3-methylbutanamido)-2-hydroxy-4-phenylbutanoyl)pyrrolidine-5-carboxamido)-3-methylbutanamido)-4-(methylthio)butanamido)-3-(1H-imidazol-4-yl)propa::CHEMBL233025::H-Pro-Gln-Val-Apns-Pro-Val-Met-His-OH::H-Pro-Gln-Val-Leu-Pro-Val-Met-His-Pro-OH::KNI-10161
SMILES: CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1)C(C)C)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O
InChI Key: InChIKey=VJXOFNUAQFCRFQ-QTCJXMFWSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human T-lymphotropic virus 1) | BDBM50227057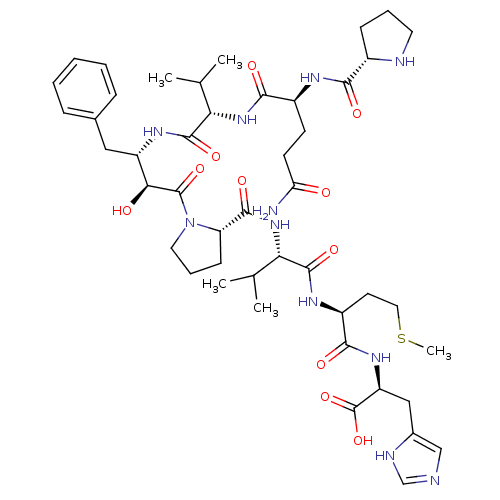 ((S)-2-((S)-2-((S)-2-((S)-1-((2S,3S)-3-((S)-2-((S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HTLV1 protease1 | Bioorg Med Chem 16: 5795-802 (2008) Article DOI: 10.1016/j.bmc.2008.03.055 BindingDB Entry DOI: 10.7270/Q2VT1RWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human T-cell leukemia virus type I) | BDBM50227057 ((S)-2-((S)-2-((S)-2-((S)-1-((2S,3S)-3-((S)-2-((S)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HTLV1 protease L40I mutant | Bioorg Med Chem 16: 6880-90 (2008) Article DOI: 10.1016/j.bmc.2008.05.052 BindingDB Entry DOI: 10.7270/Q25B059Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human T-cell leukemia virus type I protease (Human T-cell leukemia virus 1 (strain Japan ATK-1 ...) | BDBM50227057 ((S)-2-((S)-2-((S)-2-((S)-1-((2S,3S)-3-((S)-2-((S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HTLV1 protease L40I mutant expressed in Escherichia coli BL21(DE3)pLysS | Bioorg Med Chem Lett 18: 366-70 (2008) Article DOI: 10.1016/j.bmcl.2007.10.066 BindingDB Entry DOI: 10.7270/Q2N58N78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||