

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50235019 CHEMBL4067013
SMILES: CC(C)n1cnc2c(Nc3cc(Cl)cc(NC(C)=O)c3)nc(N[C@H]3CCCC[C@H]3N)nc12
InChI Key: InChIKey=ZYFRJAWGLQYBTJ-MSOLQXFVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase CLK1/CLK4 (Homo sapiens (Human)) | BDBM50235019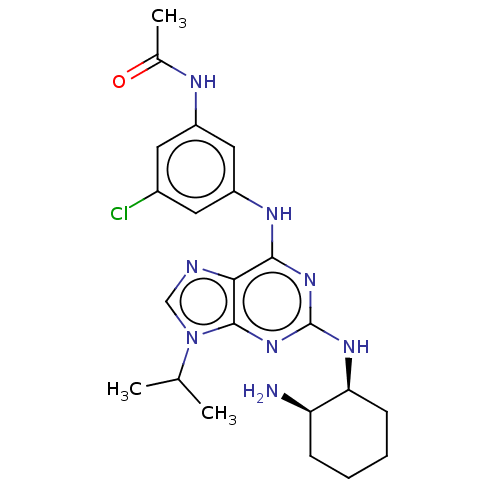 (CHEMBL4067013) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of human CLK1 using substrate ERMRPRKRQGSVRRRV in presence of [gamma-33P]ATP after 40 mins by scintillation counter method | Bioorg Med Chem Lett 27: 406-412 (2017) Article DOI: 10.1016/j.bmcl.2016.12.056 BindingDB Entry DOI: 10.7270/Q27946ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 1 (CDK1) (Homo sapiens (Human)) | BDBM50235019 (CHEMBL4067013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of human CDK1/cyclinB using Histone H1 as substrate in presence of [gamma-33P]ATP after 40 mins by scintillation counter method | Bioorg Med Chem Lett 27: 406-412 (2017) Article DOI: 10.1016/j.bmcl.2016.12.056 BindingDB Entry DOI: 10.7270/Q27946ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1/CLK4 (Homo sapiens (Human)) | BDBM50235019 (CHEMBL4067013) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of human CLK4 using YRRAAVPPSPSLSRHSSPHQS(p)EDEEE as substrate in presence of [gamma-33P]ATP after 40 mins by scintillation counter method | Bioorg Med Chem Lett 27: 406-412 (2017) Article DOI: 10.1016/j.bmcl.2016.12.056 BindingDB Entry DOI: 10.7270/Q27946ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK4/Cyclin D3 (Homo sapiens (Human)) | BDBM50235019 (CHEMBL4067013) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of human CDK4/cyclinD3 using Histone H1 as substrate in presence of [gamma-33P]ATP after 40 mins by scintillation counter method | Bioorg Med Chem Lett 27: 406-412 (2017) Article DOI: 10.1016/j.bmcl.2016.12.056 BindingDB Entry DOI: 10.7270/Q27946ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK2 (Homo sapiens (Human)) | BDBM50235019 (CHEMBL4067013) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of human CLK2 using YRRAAVPPSPSLSRHSSPHQS(p)EDEEE as substrate in presence of [gamma-33P]ATP after 40 mins by scintillation counter method | Bioorg Med Chem Lett 27: 406-412 (2017) Article DOI: 10.1016/j.bmcl.2016.12.056 BindingDB Entry DOI: 10.7270/Q27946ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK6/CycD3 (Homo sapiens (Human)) | BDBM50235019 (CHEMBL4067013) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclinD3 using Histone H1 as substrate in presence of [gamma-33P]ATP after 40 mins by scintillation counter method | Bioorg Med Chem Lett 27: 406-412 (2017) Article DOI: 10.1016/j.bmcl.2016.12.056 BindingDB Entry DOI: 10.7270/Q27946ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK3 (Homo sapiens (Human)) | BDBM50235019 (CHEMBL4067013) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of human CLK3 using ERMRPRKRQGSVRRRV as substrate in presence of [gamma-33P]ATP after 40 mins by scintillation counter method | Bioorg Med Chem Lett 27: 406-412 (2017) Article DOI: 10.1016/j.bmcl.2016.12.056 BindingDB Entry DOI: 10.7270/Q27946ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1/CLK4 (Homo sapiens (Human)) | BDBM50235019 (CHEMBL4067013) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of CLK mediated SF3B1 activation in human SK-MEL-2 cells assessed as MDM2-pre mRNA exon skipping after 4 hrs by luciferase reporter gene a... | Bioorg Med Chem Lett 27: 406-412 (2017) Article DOI: 10.1016/j.bmcl.2016.12.056 BindingDB Entry DOI: 10.7270/Q27946ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||