

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50241582 (S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-tetrahydro-2H-pyran-2-yloxy)-5-hydroxy-2-(4-hydroxyphenyl)chroman-4-one::CHEMBL451532::cid_25075::cid_442428::naringin
SMILES: C[C@@H]1O[C@@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2Oc2cc(O)c3C(=O)C[C@H](Oc3c2)c2ccc(O)cc2)[C@H](O)[C@H](O)[C@H]1O
InChI Key: InChIKey=DFPMSGMNTNDNHN-ZPHOTFPESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G protein-activated inward rectifier potassium channel 4 (Homo sapiens (Human)) | BDBM50241582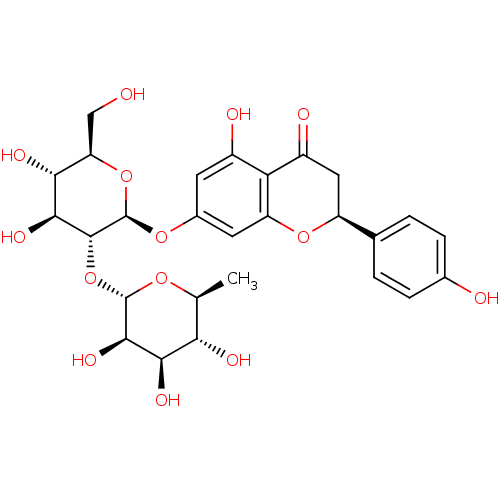 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Activation of recombinant human GIRK1/4 expressed in Xenopus oocytes by two-electrode voltage clamp method | Bioorg Med Chem Lett 23: 5195-8 (2013) Article DOI: 10.1016/j.bmcl.2013.07.002 BindingDB Entry DOI: 10.7270/Q28K7BG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-activated inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Activation of recombinant human GIRK1/2 expressed in Xenopus oocytes by two-electrode voltage clamp method | Bioorg Med Chem Lett 23: 5195-8 (2013) Article DOI: 10.1016/j.bmcl.2013.07.002 BindingDB Entry DOI: 10.7270/Q28K7BG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ubiquitin-conjugating enzyme E2 N (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2X34VX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ubiquitin-conjugating enzyme E2 N (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 5.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2SB4462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bfl-1 (Mus musculus (Mouse)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q25D8Q92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bfl-1 (Mus musculus (Mouse)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q25D8Q92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Homo sapiens (human) recombinant GSK3beta after 30 min by Kinase-Glo assay | Citation and Details Article DOI: 10.1007/s00044-012-0353-y BindingDB Entry DOI: 10.7270/Q29Z97T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ubiquitin-conjugating enzyme E2 N (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 6.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2SB4462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es Curated by ChEMBL | Assay Description Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... | Bioorg Med Chem 21: 1944-51 (2013) Article DOI: 10.1016/j.bmc.2013.01.033 BindingDB Entry DOI: 10.7270/Q2HQ419G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Minas Gerais Curated by ChEMBL | Assay Description Inhibition of aromatase | J Nat Prod 71: 1082-4 (2008) Article DOI: 10.1021/np800098f BindingDB Entry DOI: 10.7270/Q2ZS2XF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B | J Nat Prod 69: 1572-6 (2006) Article DOI: 10.1021/np0601861 BindingDB Entry DOI: 10.7270/Q2B8591J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonbuk National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate pretreated for 10 mins followed by substrate addition measured after 20 mins by spectrop... | Bioorg Med Chem Lett 27: 2274-2280 (2017) Article DOI: 10.1016/j.bmcl.2017.04.054 BindingDB Entry DOI: 10.7270/Q2ZK5K3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ubiquitin-conjugating enzyme E2 N (Homo sapiens (Human)) | BDBM50241582 ((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2X34VX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||