

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50252093 6-hydroxybenzo[d][1,3]oxathiol-2-one::CHEMBL442687::cid_72139
SMILES: Oc1ccc2sc(=O)oc2c1
InChI Key: InChIKey=SLYPOVJCSQHITR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| large T antigen (Simian virus 40) | BDBM50252093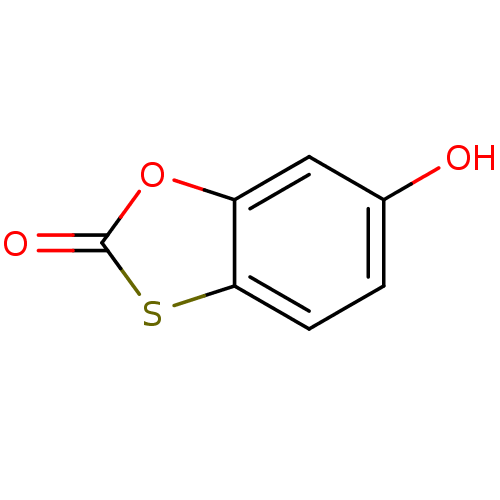 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a |
Southern Research Specialized Biocontainment Screening Center Curated by PubChem BioAssay | Assay Description Southern Research's Specialized Biocontainment Screening Center (SRSBSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Librarie... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2ZK5F47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal alkaline phosphatase (Homo sapiens (Human)) | BDBM50252093 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | n/a | n/a | 8.33E+3 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q29G5K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase placental-like (Homo sapiens (Human)) | BDBM50252093 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2SJ1J25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50252093 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2H130G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50252093 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 9.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B after 20 mins using 50 uM kynuramine as substrate by fluorescence spectrophotometry | Bioorg Med Chem Lett 26: 1200-4 (2016) BindingDB Entry DOI: 10.7270/Q2154JWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| FXN frataxin (Aspergillus niger) | BDBM50252093 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | n/a | n/a | >5.96E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute, TS... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2PK0DN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252093 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 assessed as activity of carbonic anhydrase 2 esterase activity against 4-nitrophenyl acetate | Bioorg Med Chem Lett 18: 3938-41 (2008) Article DOI: 10.1016/j.bmcl.2008.06.024 BindingDB Entry DOI: 10.7270/Q2ZW1KQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50252093 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 9.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A after 20 mins using 50 uM kynuramine as substrate by fluorescence spectrophotometry | Bioorg Med Chem Lett 26: 1200-4 (2016) BindingDB Entry DOI: 10.7270/Q2154JWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpi (Rattus norvegicus (Rat)) | BDBM50252093 (6-hydroxybenzo[d][1,3]oxathiol-2-one | CHEMBL44268...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2DB809V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||