

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50258622 CHEMBL4097898
SMILES: COc1ccncc1CCCNCc1ccc2ccc(N)nc2c1
InChI Key: InChIKey=IQPCOMIHSUSLGC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258622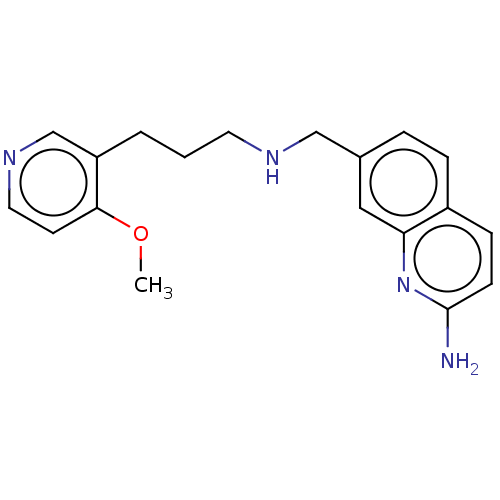 (CHEMBL4097898) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258622 (CHEMBL4097898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50258622 (CHEMBL4097898) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human eNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric-oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50258622 (CHEMBL4097898) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant bovine eNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50258622 (CHEMBL4097898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant mouse macrophage iNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||