 Found 5 hits for monomerid = 50289000
Found 5 hits for monomerid = 50289000 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-chymotrypsin
(Bos taurus (bovine)) | BDBM50289000
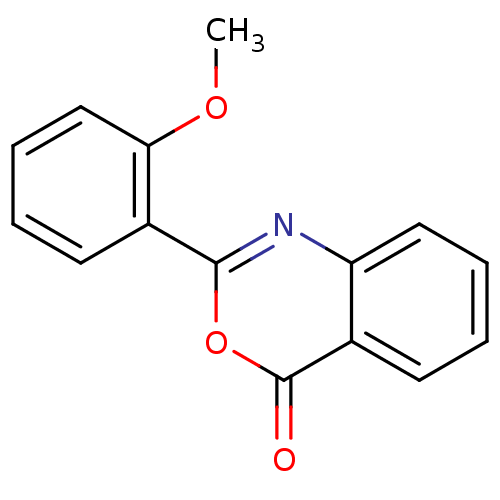
(2-(2-Methoxy-phenyl)-benzo[d][1,3]oxazin-4-one | 2...)Show InChI InChI=1S/C15H11NO3/c1-18-13-9-5-3-7-11(13)14-16-12-8-4-2-6-10(12)15(17)19-14/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
| Assay Description
The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... |
Bioorg Chem 70: 210-221 (2017)
Article DOI: 10.1016/j.bioorg.2017.01.001
BindingDB Entry DOI: 10.7270/Q2QR4W00 |
More data for this
Ligand-Target Pair | |
Complement C1r
(Homo sapiens (Human)) | BDBM50289000

(2-(2-Methoxy-phenyl)-benzo[d][1,3]oxazin-4-one | 2...)Show InChI InChI=1S/C15H11NO3/c1-18-13-9-5-3-7-11(13)14-16-12-8-4-2-6-10(12)15(17)19-14/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of C1r serine protease |
Bioorg Med Chem Lett 6: 679-682 (1996)
Article DOI: 10.1016/0960-894X(96)00094-7
BindingDB Entry DOI: 10.7270/Q2P84BVF |
More data for this
Ligand-Target Pair | |
Complement C1r
(Homo sapiens (Human)) | BDBM50289000

(2-(2-Methoxy-phenyl)-benzo[d][1,3]oxazin-4-one | 2...)Show InChI InChI=1S/C15H11NO3/c1-18-13-9-5-3-7-11(13)14-16-12-8-4-2-6-10(12)15(17)19-14/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against C1r serine protease |
Bioorg Med Chem Lett 6: 679-682 (1996)
Article DOI: 10.1016/0960-894X(96)00094-7
BindingDB Entry DOI: 10.7270/Q2P84BVF |
More data for this
Ligand-Target Pair | |
Alpha-chymotrypsin
(Bos taurus (bovine)) | BDBM50289000

(2-(2-Methoxy-phenyl)-benzo[d][1,3]oxazin-4-one | 2...)Show InChI InChI=1S/C15H11NO3/c1-18-13-9-5-3-7-11(13)14-16-12-8-4-2-6-10(12)15(17)19-14/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
University of Karachi
| Assay Description
The α-chymotrypsin inhibition activity was evaluated in 50 mM Tris-HCl buffer pH 7.6 with 10 mM CaCl2. α-Chymotrypsin (bovine pancreas) at ... |
Bioorg Chem 70: 210-221 (2017)
Article DOI: 10.1016/j.bioorg.2017.01.001
BindingDB Entry DOI: 10.7270/Q2QR4W00 |
More data for this
Ligand-Target Pair | |
Complement C1r subcomponent
(Homo sapiens (Human)) | BDBM50289000

(2-(2-Methoxy-phenyl)-benzo[d][1,3]oxazin-4-one | 2...)Show InChI InChI=1S/C15H11NO3/c1-18-13-9-5-3-7-11(13)14-16-12-8-4-2-6-10(12)15(17)19-14/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against C1r serine protease |
Bioorg Med Chem Lett 6: 679-682 (1996)
Article DOI: 10.1016/0960-894X(96)00094-7
BindingDB Entry DOI: 10.7270/Q2P84BVF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data