

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50292636 4-hexyl resorcinol::ACRISORCIN::CHEMBL443605
SMILES: CCCCCCc1ccc(O)cc1O
InChI Key: InChIKey=WFJIVOKAWHGMBH-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosinase (Homo sapiens (Human)) | BDBM50292636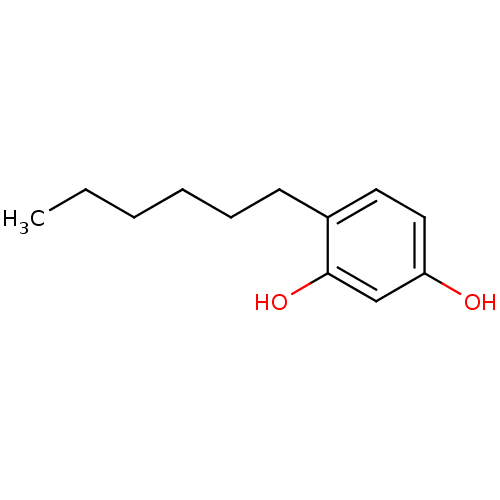 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis | Bioorg Med Chem 23: 6650-8 (2015) BindingDB Entry DOI: 10.7270/Q2DR2X9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Cagliari Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosine kinase assessed as oxidation of 0.25 mM L-3,4-dihydroxyphenylalanine | Bioorg Med Chem Lett 19: 36-9 (2008) Article DOI: 10.1016/j.bmcl.2008.11.020 BindingDB Entry DOI: 10.7270/Q2445MB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 15-lipoxygenase (Homo sapiens (Human)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 15-LO in human PMNL using arachidonic acid as substrate preincubated for 15 mins | Eur J Med Chem 67: 269-79 (2013) Article DOI: 10.1016/j.ejmech.2013.06.039 BindingDB Entry DOI: 10.7270/Q2XW4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as dopachrome formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 24: 122-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.063 BindingDB Entry DOI: 10.7270/Q2NZ8BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate after 10 mins | Eur J Med Chem 67: 269-79 (2013) Article DOI: 10.1016/j.ejmech.2013.06.039 BindingDB Entry DOI: 10.7270/Q2XW4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 12-Lipoxygenase (12-LOX) (Homo sapiens (Human)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 12-LO in human PMNL using arachidonic acid preincubated for 15 mins | Eur J Med Chem 67: 269-79 (2013) Article DOI: 10.1016/j.ejmech.2013.06.039 BindingDB Entry DOI: 10.7270/Q2XW4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using TBC as substrate by spectrophotometric method | Bioorg Med Chem Lett 29: 313-316 (2019) Article DOI: 10.1016/j.bmcl.2018.11.029 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50292636 (4-hexyl resorcinol | ACRISORCIN | CHEMBL443605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-LO in human PMNL using arachidonic acid as substrate preincubated for 15 mins | Eur J Med Chem 67: 269-79 (2013) Article DOI: 10.1016/j.ejmech.2013.06.039 BindingDB Entry DOI: 10.7270/Q2XW4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||