

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50296314 (3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carboxylate::CHEMBL549577
SMILES: O=C(O[C@H]1CN2CCC1CC2)C1c2ccccc2Oc2ccccc12
InChI Key: InChIKey=DAIWBMZPSVQRCH-IBGZPJMESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic receptor M1 (Bos taurus) | BDBM50296314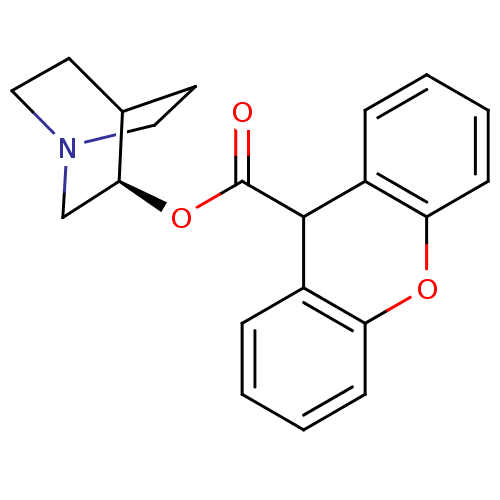 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatum | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50296314 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50296314 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 31: 1463-6 (1988) BindingDB Entry DOI: 10.7270/Q2DV1KGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296314 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 receptor expressed in CHOK1 cells by microplate scintillation counting | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296314 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296314 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M2 receptor expressed in CHOK1 cells by microplate scintillation counting | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||