

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50296315 (3R)-1-Azabicyclo[2.2.2]oct-3-yl 10,11-dihydro-5H-dibenzo-[a,d][7]annulene-5-carboxylate::CHEMBL551525
SMILES: O=C(O[C@H]1CN2CCC1CC2)C1c2ccccc2CCc2ccccc12
InChI Key: InChIKey=DYANBEYXYZKCOS-NRFANRHFSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296315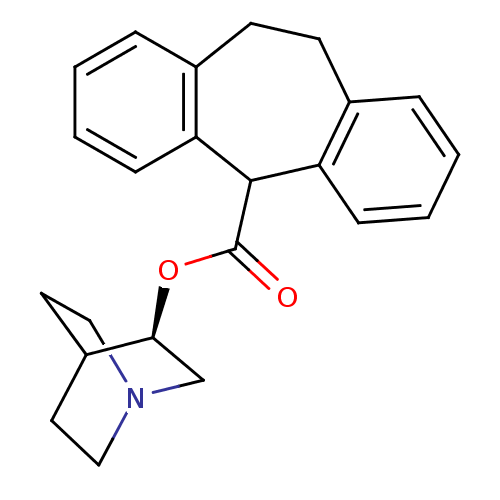 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl 10,11-dihydro-5H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M2 receptor expressed in CHOK1 cells by microplate scintillation counting | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296315 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl 10,11-dihydro-5H-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M1 receptor expressed in CHOK1 cells by microplate scintillation counting | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296315 ((3R)-1-Azabicyclo[2.2.2]oct-3-yl 10,11-dihydro-5H-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||