

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50333262 4-(2-(4-chloro-3-fluorophenylamino)-2-oxoacetamido)-1,2,2,6,6-pentamethylpiperidine 1-oxide::CHEMBL1645130
SMILES: CC1(C)CC(CC(C)(C)[N+]1(C)[O-])NC(=O)C(=O)Nc1ccc(Cl)c(F)c1
InChI Key: InChIKey=DNJQCBFBABSUGD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50333262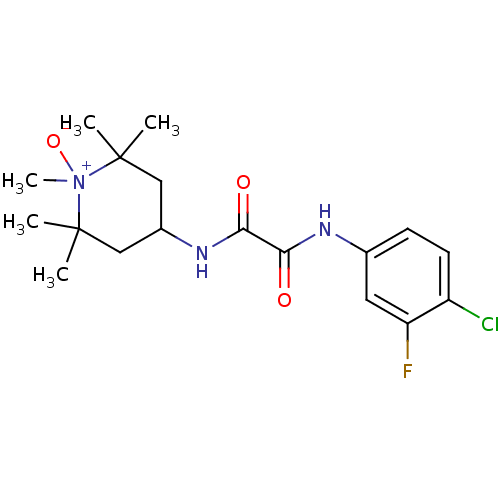 (4-(2-(4-chloro-3-fluorophenylamino)-2-oxoacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Binding affinity to HIV1 YU2 gp120 by isothermal titration calorimetry assay | Bioorg Med Chem 19: 91-101 (2011) Article DOI: 10.1016/j.bmc.2010.11.049 BindingDB Entry DOI: 10.7270/Q25H7GHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50333262 (4-(2-(4-chloro-3-fluorophenylamino)-2-oxoacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of HIV1 YU2 gp120 binding to CD4 expressing Cf2Th-CD4/CCR5 cells assessed as inhibition of viral infection after 48 hrs | Bioorg Med Chem 19: 91-101 (2011) Article DOI: 10.1016/j.bmc.2010.11.049 BindingDB Entry DOI: 10.7270/Q25H7GHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||