

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50361251 CHEMBL1934893
SMILES: CC(=O)Nc1ccc(NC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)NO)cc1
InChI Key: InChIKey=WLACYMKJIFQEMV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50361251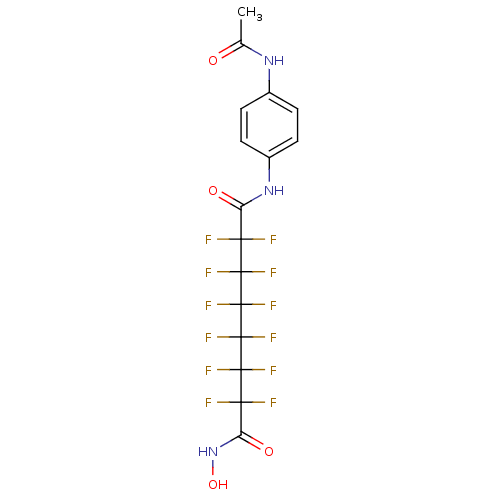 (CHEMBL1934893) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Inhibitory potency against Varicella zoster virus ribonucleotide reductase | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50361251 (CHEMBL1934893) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Darmstadt Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 27: 1508-1512 (2017) Article DOI: 10.1016/j.bmcl.2017.02.050 BindingDB Entry DOI: 10.7270/Q2JD502H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50361251 (CHEMBL1934893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50361251 (CHEMBL1934893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Boc-L-Lys(trifluoroacetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361251 (CHEMBL1934893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase-like amidohydrolase (Alcaligenes sp. (strain DSM 11172) (Bordetella sp....) | BDBM50361251 (CHEMBL1934893) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of Bordetella FB188 HDAH using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50361251 (CHEMBL1934893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC7 using Boc-L-Lys(trifluoroacetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||