

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50499822 CHEMBL3740897
SMILES: C[C@H]1Cn2c(CN1C(=O)c1cccc(c1)C(F)(F)c1n[nH]c(=O)c3ccccc13)nnc2C(C)(F)F
InChI Key: InChIKey=NWGMIELHSCQGOG-ZDUSSCGKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50499822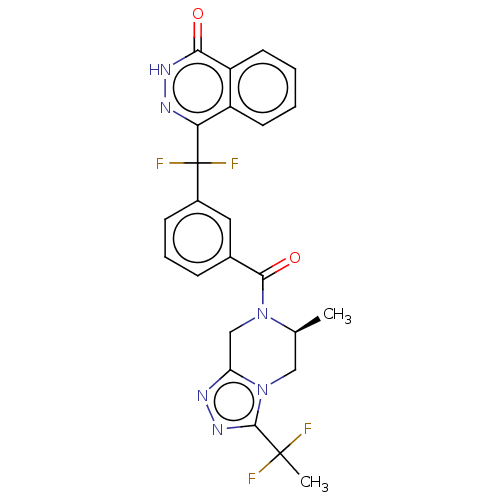 (CHEMBL3740897) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PARP in human HeLa cells assessed as induction of centrosome declustering after 48 hrs by DAPI-staining based image analysis | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PARP in human MDA-MB-157 cells assessed as induction of centrosome declustering after 48 hrs by DAPI-staining based image analysis | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human TNKS2 (667 to 1166 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by st... | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 6 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of full length human PARP6 expressed in a Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by streptavidi... | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human PARP2 (2 to 583 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by strep... | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human TNKS1 (1001 to 1327 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by s... | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PARP in human HCC1806 cells assessed as induction of centrosome declustering after 48 hrs by DAPI-staining based image analysis | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein mono-ADP-ribosyltransferase PARP3 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PARP3 (unknown origin) using activated DNA as substrate after 1 hr by streptavidin-horseradish peroxidase-based luminescence assay | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PARP in human MCF7 cells assessed as induction of centrosome declustering after 48 hrs by DAPI-staining based image analysis | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of full length human PARP1 expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by streptavidin-... | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tankyrase 1/2 (Homo sapiens (Human)) | BDBM50499822 (CHEMBL3740897) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of TNSK1/2-activated Wnt pathway in human DLD-1 TOPFlash/EF1a Renilla reporter cells after 24 hrs by Steady-Glo Luciferase assay | Bioorg Med Chem Lett 25: 5743-7 (2015) Article DOI: 10.1016/j.bmcl.2015.10.079 BindingDB Entry DOI: 10.7270/Q2WH2T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||