

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50521226 CHEMBL4471545
SMILES: CS(=O)(=O)C(c1nnc(CNS(N)(=O)=O)o1)c1nc2ccc(cc2s1)-c1ccccc1
InChI Key: InChIKey=JRKXXINBXZAUPN-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatic Lipase (HL) (Homo sapiens (Human)) | BDBM50521226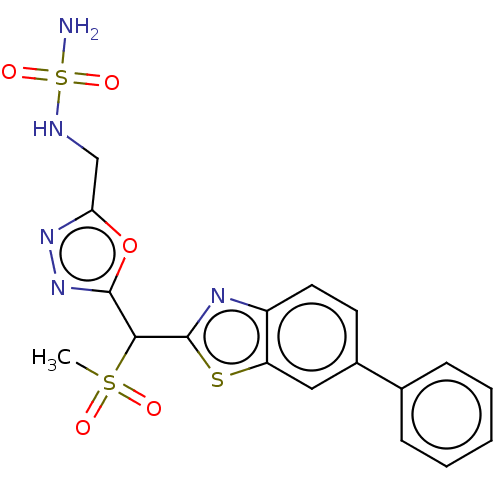 (CHEMBL4471545) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human HL expressed in COS7 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by subs... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50521226 (CHEMBL4471545) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50521226 (CHEMBL4471545) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50521226 (CHEMBL4471545) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human LPL expressed in COS7 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by sub... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50521226 (CHEMBL4471545) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic lipase (Homo sapiens (Human)) | BDBM50521226 (CHEMBL4471545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human PL expressed in HEK293F cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by s... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||