 Found 17 hits for monomerid = 50579806
Found 17 hits for monomerid = 50579806 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50579806
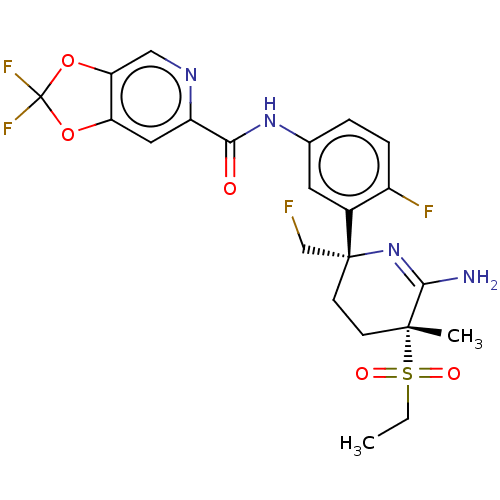
(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-JNJ962 from BACE1 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-JNJ962 from BACE2 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-imipramine from recombinant human 5-HT transporter after 60 mins by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]NKA from human recombinant NK2 receptor after 60 mins by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-pirenzepine from human recombinant muscarinic 1 receptor after 60 mins by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CCPA from human recombinant adenosine A1 receptor after 60 mins by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-AF-DX 384 from human recombinant muscarinic 2 receptor after 60 mins by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch-clamp assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reversible inhibition of human CYP2C19 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reversible inhibition of human CYP2D6 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reversible inhibition of human CYP1A2 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin D incubated for 3.5 hrs by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in human SNKBE2 cells expressing wild type APP695 assessed as reduction in amyloid beta 42 secretion incubated for 18 hrs by sand... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Mus musculus (Mouse)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE2 in mouse MIN6 cells expressing TMEM27 assessed as reduction in TMEM27 secretion incubated for 24 hrs by MSD electrochemiluminesce... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys-Met/Asn-Leu mutant-derived peptide as substrate by FRET assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50579806

(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE2 (unknown origin) using APP harboring Swedish Lys-Met/Asn-Leu mutant-derived peptide as substrate incubated for 2 hrs by FRET assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data