

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Oc1ccc(cc1)-c1ccc2c(NC(=O)C3CC3)n[nH]c2c1
InChI Key: InChIKey=RXQXACYMRVINQU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8370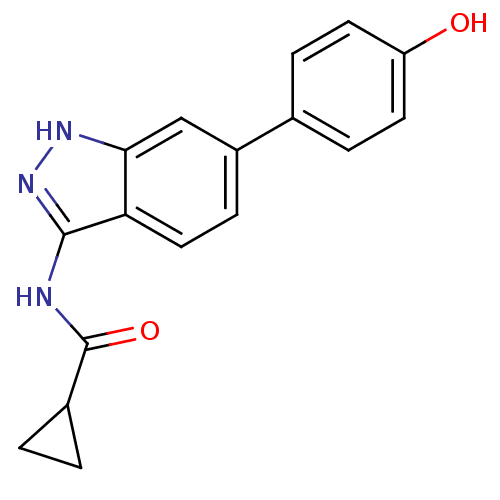 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 3055-7 (2003) Article DOI: 10.1016/s0960-894x(03)00645-0 BindingDB Entry DOI: 10.7270/Q26T0JVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 3059-62 (2003) Article DOI: 10.1016/s0960-894x(03)00646-2 BindingDB Entry DOI: 10.7270/Q2348HKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant GSK3-beta | J Med Chem 51: 2062-77 (2008) Article DOI: 10.1021/jm7009765 BindingDB Entry DOI: 10.7270/Q20Z744C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate pretreated for 15 mins followed by substrate addition measured after 1 hr | ACS Med Chem Lett 8: 1093-1098 (2017) Article DOI: 10.1021/acsmedchemlett.7b00296 BindingDB Entry DOI: 10.7270/Q28S4SDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Inhibition of AAK1 (unknown origin) using Fos-Nfluc, Cfluc-kinase and rabbit reticulate lysate system after 1 hr by split luciferase assay | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0-ATP synthase | ACS Med Chem Lett 8: 1093-1098 (2017) Article DOI: 10.1021/acsmedchemlett.7b00296 BindingDB Entry DOI: 10.7270/Q28S4SDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate pretreated for 15 mins followed by substrate addition measured after 1 hr | ACS Med Chem Lett 8: 1093-1098 (2017) Article DOI: 10.1021/acsmedchemlett.7b00296 BindingDB Entry DOI: 10.7270/Q28S4SDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate pretreated for 15 mins followed by substrate addition measured after 1 hr | ACS Med Chem Lett 8: 1093-1098 (2017) Article DOI: 10.1021/acsmedchemlett.7b00296 BindingDB Entry DOI: 10.7270/Q28S4SDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate pretreated for 15 mins followed by substrate addition measured after 1 hr | ACS Med Chem Lett 8: 1093-1098 (2017) Article DOI: 10.1021/acsmedchemlett.7b00296 BindingDB Entry DOI: 10.7270/Q28S4SDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM8370 (N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using TAMRA-ERMRPRKRQGSVRRRV-NH2 as substrate pretreated for 30 mins followed by substrate addition measured afte... | ACS Med Chem Lett 8: 1093-1098 (2017) Article DOI: 10.1021/acsmedchemlett.7b00296 BindingDB Entry DOI: 10.7270/Q28S4SDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||