

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM8580 (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2R,3S,4R,6S)-4,6-diamino-2,3-dihydroxycyclohexyl]oxy}oxane-3,4-diol::Neamine
SMILES: NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O
InChI Key: InChIKey=SYJXFKPQNSDJLI-HKEUSBCWSA-N
PDB links: 7 PDB IDs match this monomer. 3 PDB IDs contain this monomer as substructures. 8 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthrax Lethal Factor (LF) (Bacillus anthracis) | BDBM8580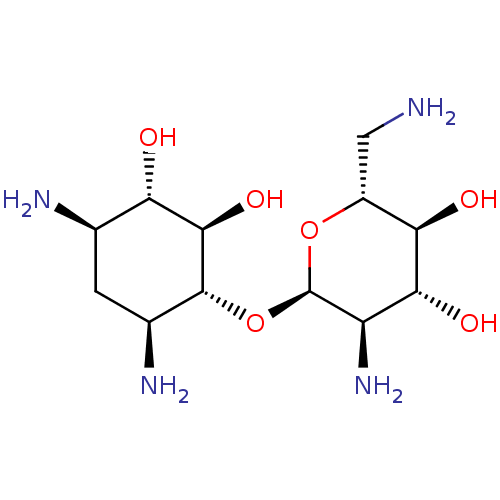 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.29E+4 | -5.89 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Hawaii Biotech Inc. | Assay Description The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o... | Bioorg Med Chem Lett 16: 5183-9 (2006) Article DOI: 10.1016/j.bmcl.2006.07.005 BindingDB Entry DOI: 10.7270/Q2VQ30WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthrax Lethal Factor (LF) (Bacillus anthracis) | BDBM8580 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.29E+4 | -5.95 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Hawaii Biotech Inc. | Assay Description The enzymatic reaction started by the addition fluorogenic peptide substrate, MAPKKide to the buffer containing LF and inhibitor compound. Cleavage o... | Bioorg Med Chem Lett 16: 1527-31 (2006) Article DOI: 10.1016/j.bmcl.2005.12.038 BindingDB Entry DOI: 10.7270/Q2FB5146 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 REV (Human immunodeficiency virus 1) | BDBM8580 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | PDB MMDB Reactome pathway KEGG B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of [3H]Rev response element binding to HIV1 biotinylated-Rev protein expressed in Escherichia coli after 15 mins by scintillation proximit... | Bioorg Med Chem Lett 29: 339-341 (2019) Article DOI: 10.1016/j.bmcl.2018.11.004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine-retinol O-acyltransferase (Homo sapiens (Human)) | BDBM8580 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of translation using highly active Escherichia coli S30 and a plasmid containing a gene expressing truncated ... | Bioorg Med Chem Lett 13: 901-3 (2003) BindingDB Entry DOI: 10.7270/Q2ZC83DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 REV (Human immunodeficiency virus 1) | BDBM8580 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | PDB MMDB Reactome pathway KEGG B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against peptide binding to the RRE RNA was determined | Bioorg Med Chem Lett 11: 591-4 (2001) BindingDB Entry DOI: 10.7270/Q2G44QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 Tat protein (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM8580 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of Tat peptide binding to HIV-1 TAR RNA | Bioorg Med Chem Lett 11: 591-4 (2001) BindingDB Entry DOI: 10.7270/Q2G44QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine-retinol O-acyltransferase (Homo sapiens (Human)) | BDBM8580 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of translation using highly active Escherichia coli S30 and a plasmid containing a gene expressing truncated ... | Bioorg Med Chem Lett 13: 901-3 (2003) BindingDB Entry DOI: 10.7270/Q2ZC83DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine-retinol O-acyltransferase (Homo sapiens (Human)) | BDBM8580 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of translation using highly active Escherichia coli S30 and a plasmid containing a gene expressing truncated ... | Bioorg Med Chem Lett 13: 901-3 (2003) BindingDB Entry DOI: 10.7270/Q2ZC83DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine-retinol O-acyltransferase (Homo sapiens (Human)) | BDBM8580 ((2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of translation using highly active Escherichia coli S30 and a plasmid containing a gene expressing truncated ... | Bioorg Med Chem Lett 13: 901-3 (2003) BindingDB Entry DOI: 10.7270/Q2ZC83DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||