

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM9142 (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,4-dihydro-2H-1-benzopyran-4-yl]carbamoyl}butyl]-4-{2-[5-(pyridin-4-yl)furan-2-yl]propan-2-yl}-N-(2,2,2-trifluoroethyl)piperazine-2-carboxamide::P3 biaryl pyridylfuran analog 3
SMILES: CC(C)(N1CCN(C[C@@H](O)C[C@@H](Cc2ccccc2)C(=O)N[C@@H]2[C@H](O)COc3ccccc23)[C@@H](C1)C(=O)NCC(F)(F)F)c1ccc(o1)-c1ccncc1
InChI Key: InChIKey=DYYJWDSYFDXNOW-FLOLVYIHSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM9142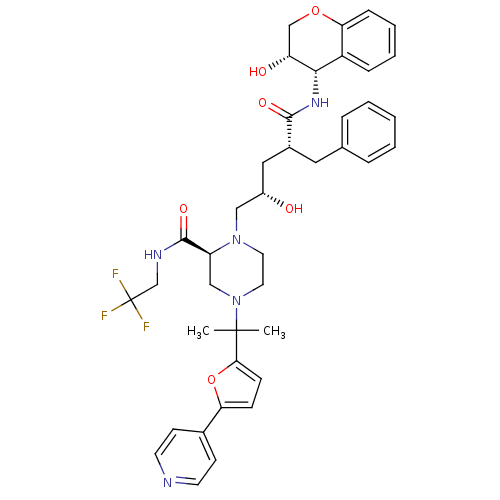 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2573-6 (2003) Article DOI: 10.1016/s0960-894x(03)00474-8 BindingDB Entry DOI: 10.7270/Q2TD9VJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease Mutant Q-60C (Human immunodeficiency virus type 1) | BDBM9142 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2569-72 (2003) Article DOI: 10.1016/s0960-894x(03)00475-x BindingDB Entry DOI: 10.7270/Q2PN93V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease Mutant V-18C (Human immunodeficiency virus type 1) | BDBM9142 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2569-72 (2003) Article DOI: 10.1016/s0960-894x(03)00475-x BindingDB Entry DOI: 10.7270/Q2PN93V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease Mutant K-60C (Human immunodeficiency virus type 1) | BDBM9142 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2569-72 (2003) Article DOI: 10.1016/s0960-894x(03)00475-x BindingDB Entry DOI: 10.7270/Q2PN93V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||